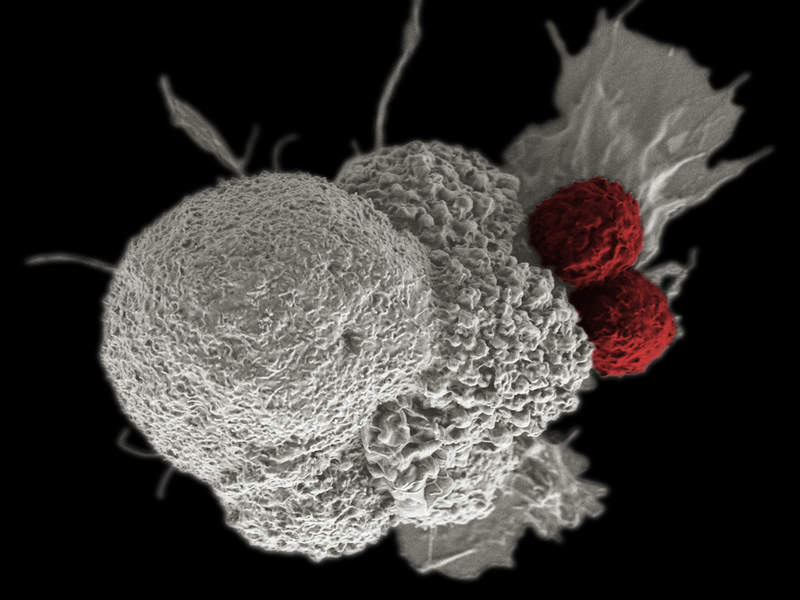
National Health Service (NHS) England has reached an agreement with Gilead Sciences to provide the pharmaceutical company’s chimeric antigen receptor T-cell (CAR-T) therapy Yescarta (axicabtagene ciloleucel) for adults with large cell lymphoma.
Yescarta uses the patient’s own immune system to fight against cancer, and will be available for patients whose cancer has returned or stopped responding to prior treatment.

Discover B2B Marketing That Performs
Combine business intelligence and editorial excellence to reach engaged professionals across 36 leading media platforms.
The agreement will enable the NHS to offer the personalised medicine for up to 200 patients per year.
NHS England chief executive Simon Stevens said: “Thanks to investment in game-changing techniques like CAR-T, the NHS is at the forefront of providing a new wave of personalised treatments that are individually tailored to patients.
“CAR-T cell therapy is one of the most promising new treatments in a generation for lymphoma and leukaemia, and NHS patients will now be among the first in the world to benefit.”
Set to be available over the coming weeks, Yescarta will be initially offered at NHS hospitals in Birmingham, Bristol, London, Manchester and Newcastle.

US Tariffs are shifting - will you react or anticipate?
Don’t let policy changes catch you off guard. Stay proactive with real-time data and expert analysis.
By GlobalDataAt its full list price, the treatment would have cost approximately £300,000 per patient. However, a commercial agreement with the manufacturer Gilead has enabled the UK’s pharma pricing regulator the National Institute of Health and Care Excellence (NICE) to approve its inclusion in NHS England’s Cancer Drugs Fund.
Gilead Sciences UK and Ireland general manager Hilary Hutton-Squire said: “This is an important day for patients with lymphoma who may have run out of effective treatment options leaving them just months to live.
“We are delighted to have been able to reach an agreement by working closely with NHS England and NICE which allows us to bring this new generation of personalised cancer treatment to patients.”
Last month, NHS England entered into a similar deal with Novartis to fund the use of Kymriah, also a CAR-T therapy, to treat refractory acute lymphoblastic leukaemia (ALL) in children and young adults aged below 25 years.




