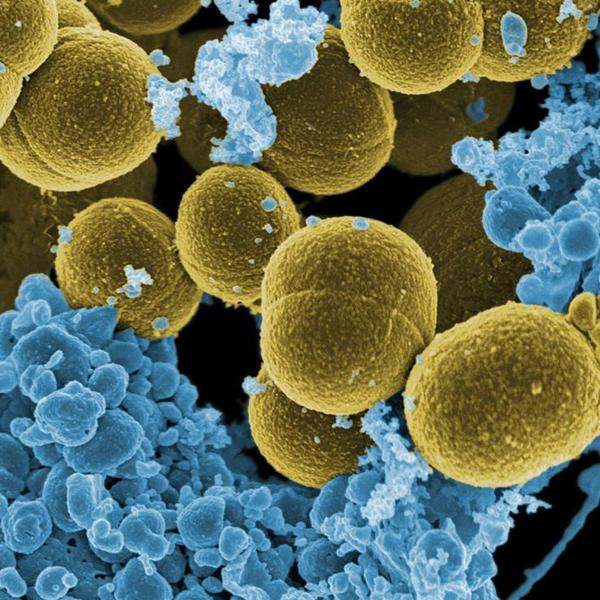
The National Institutes of Health (NIH) division National Institute of Allergy and Infectious Diseases (NIAID) has announced support to US clinical sites that are joining two clinical trials being conducted to treat infections caused by Pseudomonas aeruginosa (P. aeruginosa) and Staphylococcus aureus (S. aureus).
The global trials are being supported by AztraZeneca’s global biologics research and development arm MedImmune in alliance with Belgian Innovative Medicines Initiative Joint Undertaking and the Combatting Antimicrobial Resistance in Europe (COMBACTE) consortium.

Discover B2B Marketing That Performs
Combine business intelligence and editorial excellence to reach engaged professionals across 36 leading media platforms.
A clinical research consortium called Antibacterial Resistance Leadership Group (ARLG) will support the 15 US sites that will enrol around 30 adults who are on mechanical ventilation in intensive care units.
Two randomised, placebo-controlled, double-blind trials, EVADE and SAATELLITE, will be performed at the sites to assess MedImmune’s investigational candidates MEDI3902 and suvratoxumab, respectively.
Both the candidates are monoclonal antibodies being developed as preventive therapies to be given in conjunction with conventional antibiotic treatment.
NIAID director Anthony Fauci said: “It is becoming increasingly common for hospitalised patients, especially those with weakened immune systems, to develop severe, hard-to-treat bacterial infections.

US Tariffs are shifting - will you react or anticipate?
Don’t let policy changes catch you off guard. Stay proactive with real-time data and expert analysis.
By GlobalData“These clinical trials testing monoclonal antibodies as novel preventive therapies are part of a global collaborative effort to explore innovative ways to mitigate the threat of antimicrobial resistance.”
The EVADE trial will include patients colonised with P. aeruginosa bacteria in the lower respiratory tract but display no signs of pneumonia infection. After an intravenous (IV) infusion of MEDI3902, patients will be monitored for pneumonia incidence for 21 days and followed up for an additional 28 days.
Subjects colonised with S. aureus in the lower respiratory tract but not having infection associated with the specific bacteria will be given IV suvratoxumab in the SAATELLITE trial.
During this trial, participants will be assessed for incidents of S. aureus-related pneumonia for 30 days, and a subsequent 160-day follow up.




