On 15 August 2025, Novo Nordisk’s Wegovy (semaglutide 2.4mg) received accelerated approval from the US Food and Drug Administration for the treatment of metabolic-associated steatohepatitis (MASH) in adults with moderate-to-advanced fibrosis (excessive scar tissue in the liver). This is the second FDA-approved therapy for MASH following Madrigal Pharmaceuticals’ Rezdiffra (resmetirom) in 2024, and the third indication overall for Wegovy, which is already approved for obesity and cardiovascular risk reduction.
Wegovy is a GLP-1 (glucagon-like peptide 1) receptor agonist that addresses systemic metabolic dysfunction while improving liver histology, offering a differentiated approach compared with Rezdiffra, which primarily targets liver fat accumulation and fibrosis without systemic effects.
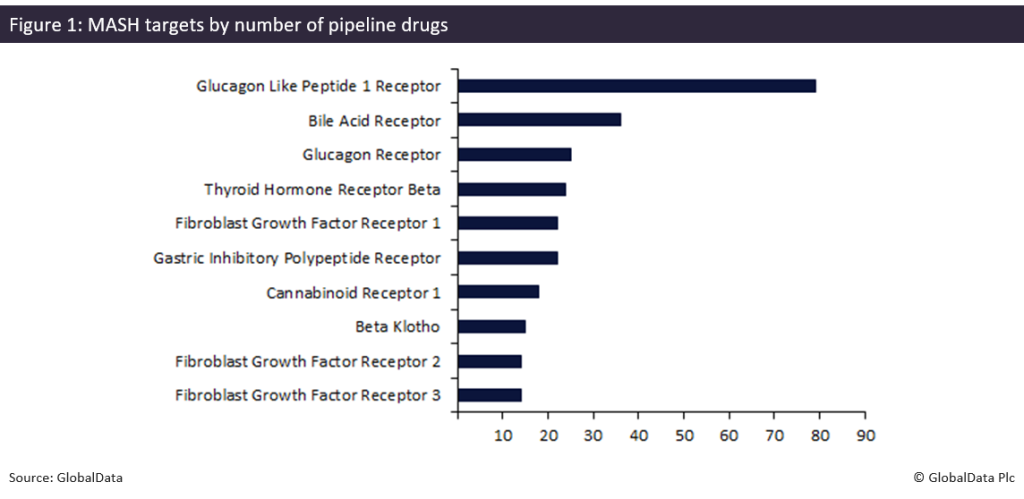
The MASH therapeutic landscape in the US is at an inflection point. While only two therapies have gained approval, the broader pipeline paints a picture of strong research and development activity and strategic focus on specific targets. As shown in Figure 1, GLP-1 receptors dominate the pipeline with 79 drugs — far exceeding other targets such as bile acid or glucagon receptors — demonstrating both the scientific and commercial focus on this target.
Wegovy’s approval opens a new metabolic liver segment for GLP-1 therapies, positioning Novo Nordisk as a first mover in combining systemic metabolic benefits with liver histology improvements. Adoption is expected to be accelerated by the overlap with obesity and diabetes populations, encouraging multidisciplinary prescribing from hepatologists, endocrinologists and cardiologists.
The strong pipeline shown in Figure 1, with 79 drugs targeting the GLP-1 receptor in the MASH pipeline, underscores the strategic advantage of GLP-1 receptor targeting, reflecting robust investment and development activity in this class. Wegovy’s approval not only validates this but also sets a benchmark for future MASH treatments, signalling that GLP-1 therapies are likely to define the next wave of innovation and competitive activity in this indication.