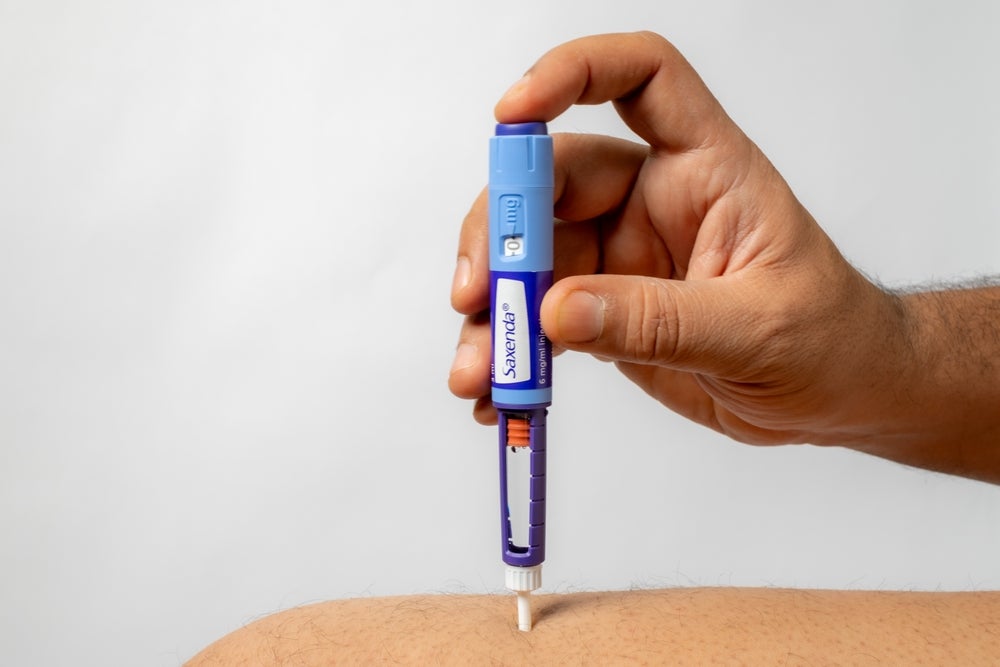
Generics company Teva Pharmaceutical has obtained approval from the US Food and Drug Administration (FDA) for its generic referencing Novo Nordisk’s previous generation of injectable weight loss drug, Saxenda (liraglutide).
This marks the first time a generic glucagon-like peptide-1 receptor agonist (GLP-1RA) medication has been approved for weight loss in the US.
Teva’s subcutaneous medication can now be administered in adult patients who are overweight or obese and have weight-related comorbidities. It is also suitable for obese patients aged between 12 and 17 years who weigh more than 60kg.
This marks Teva’s fifth first-to-market entry for a generic product this year, according to SVP and head of US commercial generics Ernie Richardsen.
Teva’s Saxenda generic approval comes amid the monumental rise in GLP-1RA sales, which have seen next-generation drugs such Eli Lilly’s Zepbound (tirzepatide) and Novo Nordisk’s Wegovy (semglutide) earn $11.3bn and $13.4bn for the companies, respectively, in 2024. These sales cemented their position in the top ten best-selling drugs of the year.
However, in releasing the longer-lasting and more effective Wegovy, Novo’s Saxenda has dwindled in market share, with analysts at GlobalData, parent company of Pharmaceutical Technology, forecasting that the drug’s sales will plummet to just $135m in 2031 – down 91% from $1.5bn peak sales in 2022.
The looming potential introduction of Lilly and Nordisk’s weight loss pills, namely orfoglipron and semaglutide, could further exacerbate Saxenda’s declining sales, with both pharma companies racing to obtain FDA approval for their versions.
However, GlobalData pharma analyst Costanza Alciati noted that generics entering the GLP-1RA market may encounter a different fate than branded Saxenda, as they are likely to capture “a big part of the patient share”.
Aliciati said: “Cost of therapy is a big unmet need in the obesity space, as branded drugs in this indication are expensive – often burdening both patients and healthcare systems.
“Not only could a liraglutide generic boost the patient population able to access GLP-1Ras, but it could also allow patients to switch from branded drugs to an older generic if a lower-efficacy product is sufficient.”
Though it is yet to be seen if liraglutide will become a favourable choice for prescribers and patients alike, Aliciati stated that the generic approval will likely have no impact on sales of Wegovy and Zepbound, as “some patients will still require higher weight loss efficacy than what is offered by liraglutide”.
Moving forward, GLP-1RA sales will likely remain on a steep upward trajectory, with analysts at GlobalData, parent company of Pharmaceutical Technology, forecasting that the drug class will be worth $125bn across the seven major markets (7MM: US, France, Germany, Italy, Spain, UK and Japan) by 2033.