Spaulding Clinical Research completed a renovation of its clinical research facility on 20 August 2010. Located in West Bend, Wisconsin, US, it is a Phase 1 Clinical Pharmacology Research facility that opened in 2008. The renovation was completed on the second floor of the facility. It created 16,700ft² of additional space and has doubled the total capacity of the facility.
The renovation was carried out in response to the rising demand for improved efficiency in Thorough QT (TQT) monitoring trials in the pharmaceutical industry. It has positioned Spaulding Clinical as the largest telemetry provider in the world. The project was financed by M&I Bank of Milwaukee and was constructed by local contractors from West Bend.
Facility
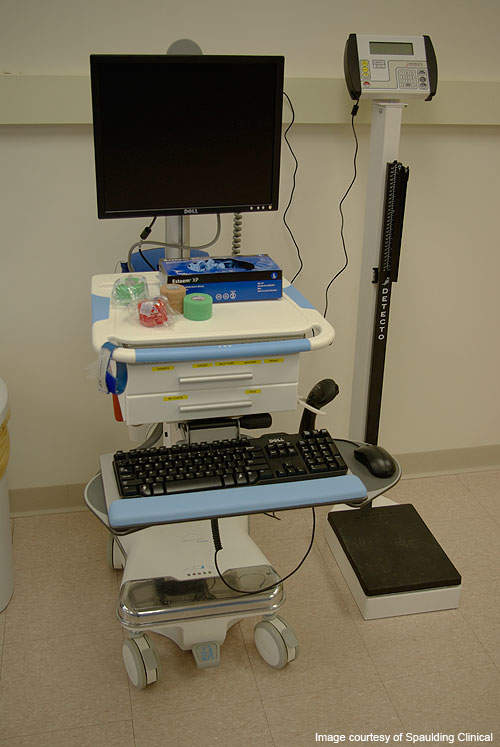
The clinical research facility is a three-storey, 200,000ft² building spread over an eight acre campus. The fully GCP-compliant facility accommodates 800ft² environmentally controlled suites each of which typically contain two beds. Each suite is equipped with private TVs, wireless internet, a closet, and private bathroom and shower facilities. Mortara Surveyor Telemetric monitoring and recording systems are installed within each suite for continuous 12-lead monitoring, trending of the patients and thorough evaluation of ECG intervals. The systems are mobile and can operate 24 hours a day.
There are a total of 48 functional telemetry beds housed on the third floor of the facility. During the renovation, the second floor was completely refitted and the total number of beds was increased to 96.
An ongoing construction project is expected to increase the beds further to 150 in the near future. The facility is equipped with unlimited expansion capabilities. Future plans also include increasing the beds across the campus to 300.
The state-of-the-art facility is outfitted with emergency equipment including a crash cart and an automatic defibrillator. The facility also includes ALPHADAS-EDC system that is designed specifically for Phase I clinical trials. There are standard and refrigerated centrifuges and a number of -30°C and -80°C freezers.
Additional capabilities within the facility include SpO2 pulse oximetry and ABPM (ambulatory blood pressure monitoring). The ground floor accommodates a secured, temperature-controlled pharmacy complete with IV infusion capability and staff pharmacist. There is also an oncology wing and space for orientation, screening and technology on the ground floor.
The third floor additionally houses computer rooms, rooms for dining, recreation and dosing, and a nurse station. The recreation room features a big screen TV with DVD player, pool table, video and board games, and books. The facility also has a 24-hour video monitoring system. All the doors within the facility are operated via a centralised automatic control.
Production
The facility conducts clinical trials and offers centralised cardiac core lab services.
Clinical conduct services performed at the facility include Thorough QT studies (TQT), cardiac safety / definitive QTc, intensive Phase I ECG trials, pharmacokinetic and pharmacodynamic characterisation of QTc, multiple rising dose, bioavailability and bioequivalence studies, drug interaction and food effect studies, first-in-human studies, pharmacokinetic analysis, protocol consultation and development, and concentration-effect modelling.
Cardiac core lab services include advice on the study design and analysis, centralised analysis and cardiologist interpretation of ECGs, QTC analysis and submission of expert reports, FDA ECG data warehouse submissions and BioQT analysis.
Process
The facility conducts clinical studies in a fully automated manner. Each subject is given a bar-coded wristband to facilitate patient identification. The bar code is scanned before starting the study procedure.
All the data is captured through a fully integrated, validated and wireless EDC system installed across the facility, including blood pressure monitoring systems, telemetry and ECG monitoring systems.
The subjects are monitored 24 hours a day and with 12 leads. Even a small change, as low as two milliseconds, on the patient can be measured. Data is processed and checked in real time at collection points and the results are returned within eight hours.