
The concept of targeted cytotoxic drug delivery to reduce systemic toxicity while increasing treatment efficacy was first introduced by Paul Ehrlich in 1913. However, it was only a century later that this concept turned into reality when the first antibody-drug conjugate (ADC) gained regulatory approval.
ADCs continue to demonstrate efficacy across different tumor types, and their use is expected to expand across indications as more suitable target antigens are identified and new and better technologies emerge.
In this article, we explore the market growth of ADCs, innovations in the ADC space, and the future of ADCs as a targeted therapy for various diseases, including cancer.
Mechanism of action of ADCs
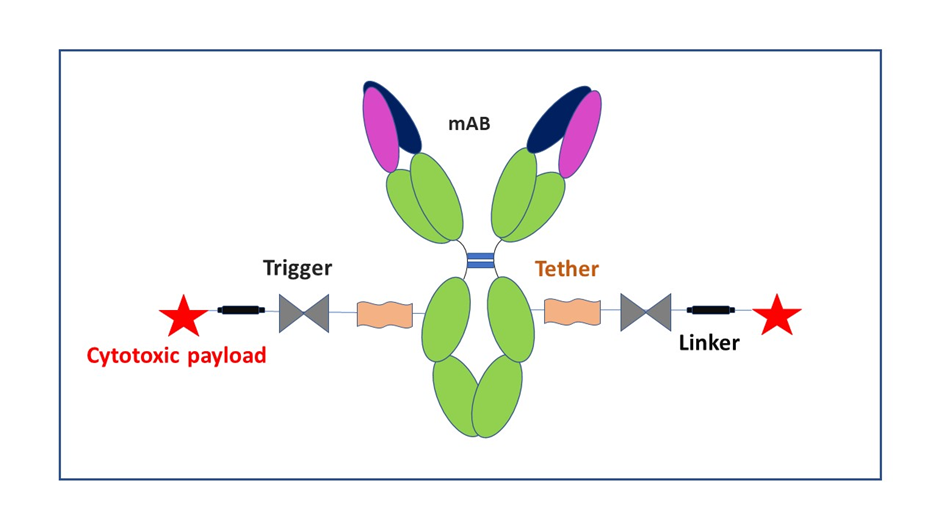
ADCs are described as a class of highly potent biologics composed of an antibody linked to a biologically active drug or cytotoxic compound. ADCs utilize monoclonal antibodies (mAbs) as the targeting component, which offer high affinity, potency, and specificity. The mAbs are conjugated to a drug or cytotoxic compound via a chemical linker.
A major benefit of ADCs is their ability to target certain proteins found on the surface of the tumor cells. This mechanism of action is different from traditional chemotherapy treatment, which can damage healthy cells. The delivery method relies on binding to a target (cancer protein or receptor) followed by the release of a cytotoxic drug into the cancer cell.
Illustration of an ADC
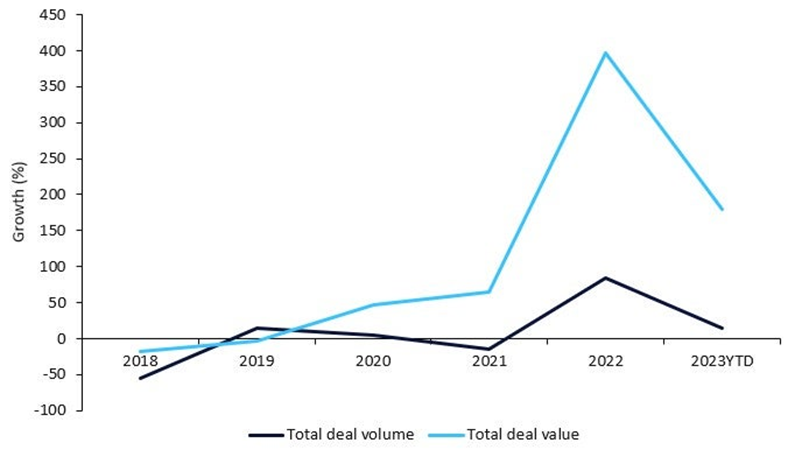
Market growth of ADCs
ADCs have shown promising results in breast, bladder, ovarian, gastric, leukemia, and lymphoma-related cancers. Further, positive clinical trial data from candidates in late-stage clinical trials have resulted in increased collaboration and investment in R&D by top players. Pharmaceutical Technology reported a 400% growth in total licensing agreement deal value from 2017 – 2022, according to GlobalData’s Pharma Intelligence Center Deals Database (1).
ADC patent pipeline
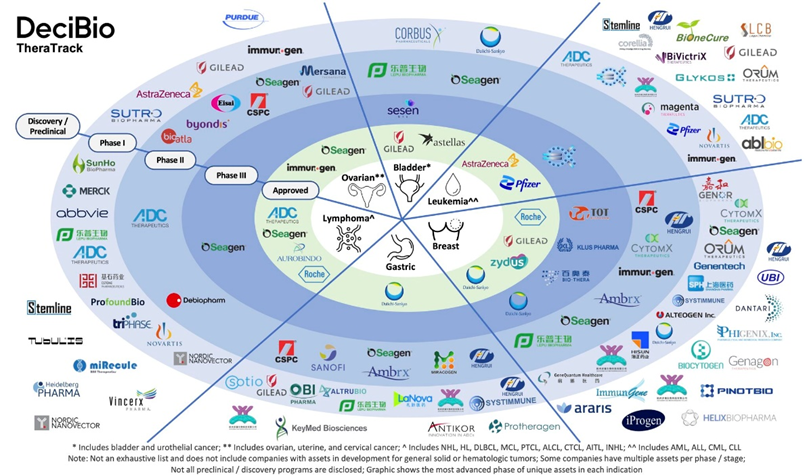
The pipeline of ADC development is being led by companies such as Sanofi, Seagen, ChemSun Biosciences, Ardeagen Corp, Starpharma Holdings Ltd, TaGeza Biopharmaceuticals Ltd, and ImmunoGen Inc.
AClinical Trials Arena commentary article published in June 2023 states that there are 12 FDA-approved ADCs, with over 170 novel ADCs (2) in the clinical stage of development, according to GlobalData’s Pharma Intelligence Center.
In terms of commercial blockbuster drugs, Daiichi Sankyo’s three lead assets, Enhertu (trastuzumab deruxtecan), datopotamab deruxtecan (Dato-Dxd), and patritumab deruxtecan (HER3-Dxd)) are expected to hold a significant chunk of the ADC market. The combined global sales of these three drugs are predicted to reach ~$13 billion by 2029, according to GlobalData’s Intelligence Centre. Other approved ADCs include Kadcyla, Blenrep, and Adcetris.
Innovation S-curve and future of ADCs
ADCs are considered an accelerating trend in GlobalData’s Technology Foresight report, which plots the innovation S-curve in the pharmaceutical industry shown below.
Popularity of ADCs accelerates
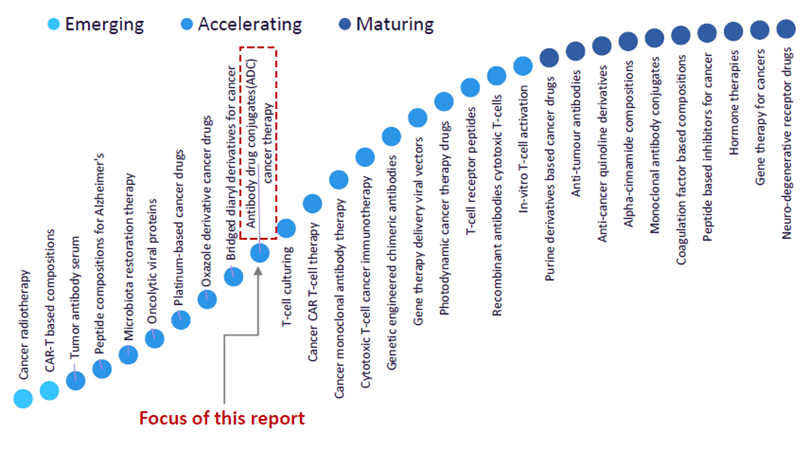
The report states that other than cancer, ADCs are also being explored as a treatment mode for autoimmune disorders and infectious diseases. Companies such as Seagen, which Pfizer recently announced an agreement to acquire (3), are industry leaders in ADC research harnessing technologies related to ADCs. Technologies include microfluidics-based technologies, streamlined expressed protein ligation technology, and ADC platforms to develop new classes of highly specific ADC linkers while identifying small molecule drugs for ADC technologies (4).
Seagen holds three ADCs in the marketplace: Adectris (brentuximab vedotin), Padcev (enfortumab vedotin), and Tivak (tistoumab vedotin), which span across both solid tumors and hematological malignancies.
In terms of ADC application areas, anti-infective therapy, anti-inflammatory therapy, and immuno-oncology are the three major fields. Oncology remains the top therapy area involving ADC drugs over the last five years – reaching a peak in 2022 with 36 licensing agreement deals.
Real-world innovation in ADCs
GlobalData’s Innovation Explorer database tracks real-world innovations related to the deployment of emerging technologies. Notable innovations in ADCs include ADCs developed by Gilead Sciences, Ambrix, Immunogen, AstraZeneca, and Daiichi Sankyo.
Gilead Sciences drug Trodelvy (sacituzumab govitecan-hziy) recently received approval from the US Food and Drug Administration (FDA) for the use of this drug. The approval was based on data where Phase-3 TROPiCS-02 research on progression-free survival and overall survival were found to be statistically significant and clinically relevant (5).
Ambryx Biopharma (Ambrx) published data from the Phase-1 portion of the ongoing Phase 1 / 2 APEX-01 trial reveals a strong and highly differentiated safety profile observed across 65 patients at all dose levels (6).
ImmunoGen has launched its cancer drug Elahere for the treatment of platinum-resistant ovarian cancer. Elahere is claimed to be a first-of-its-kind ADC applied to cancer cells and the first approved FDA drug for platinum-resistant ovarian cancer since 2014 (7).
AstraZeneca and Daiichi Sankyo have received approval from the European Union (EU) for their human epidermal growth factor receptor 2(HER2) directed ADC called Enhertu. The clinical trial results showed that Enhertu increased overall survival by more than six months compared to chemotherapy and lowered the probability of disease progression or death by 50%.
ADC Therapeutics SA’s successful ADC Zynlonta has been approved as oncology therapy for the treatment of Large B-Cell Lymphoma. The European Commission awarded a conditional marketing authorization for Zynlonta (8) (loncastuximab tesirine) to treat relapsed or refractory (r/r) diffuse large B-cell lymphoma (DLBCL).
In September 2023, the EMA’s Committee for Medical Products for Human Use had given a positive opinion about this use. Avid Bioservices and Sterling Pharma Solutions are manufacturing Zynlonta’s biological API.
Future prospects of ADCs
Advancements in Antibody-Drug Conjugate (ADC) development are reshaping the landscape of cancer therapy, focusing on enhancing efficacy and minimizing side effects. Researchers are actively investigating non-cytotoxic payloads, ranging from polyethylene glycol to antibiotics, PROTAC, LYTAC, oligonucleotides, peptides, immunomodulating agents, enzyme inhibitors, and protein ligands. Some are in the proof-of-concept stage, while others are undergoing clinical trials. Notably, the exploration of immune-stimulating agents like STING and TLR agonists is underway, strategically conjugated to antibodies to improve tolerance and reduce systemic toxicity.
Promising results are emerging from clinical trials, such as those involving ADCs like NJH395 and BDC-1001, specifically targeting HER2-positive tumors. These ADCs demonstrate low toxicity and significant clinical activity. Additionally, HSP90 inhibitors are being explored as potential ADC payloads based on encouraging preclinical model outcomes.
Companion diagnostics mark a significant stride, with biomarker identification and patient stratification based on target expression levels or predictive factors. This personalized approach optimizes patient selection for ADC treatment, improving overall clinical outcomes.
Integrating bispecific antibodies into ADCs is a strategic advancement, enabling simultaneous targeting of tumor-specific antigens and immune cells. This facilitates the immune-mediated killing of cancer cells, potentially enhancing therapeutic effects and reducing resistance.
Taking targeting specificity further, tri-specific antibodies, designed to bind to three different targets or antigens, offer the potential for precisely delivering cytotoxic payloads to tumor cells while sparing healthy tissues.
ADC clinical pipeline per therapeutic area
Accelerating ADC development with a partner of choice
Discovery, development, and manufacturing of ADCs involve a complex process that requires careful linker and antibody design, an intricate suite of bioanalytical methods, and GMP manufacturing of small molecule, linker, and mAb, culminating in the intricate step of conjugation with the mAb. This necessitates deep technical expertise and comprehensive CMC (Chemistry, Manufacturing, and Controls) capabilities in the manufacturing partner.
Global CRO/CDMO Syngene offers a highly integrated ‘one-stop-shop’ approach for ADCs underpinned by strong conceptual frameworks, experimental design, and cutting-edge technology. Our end-to-end capabilities range from pharmacokinetics and toxicology to discovery-grade conjugation and characterization, Linker payload synthesis (cytotoxic and non-cytotoxic) and optimization, and antibody discovery and engineering.
All our ADC services are available as standalone and integrated services across antibody discovery, bioconjugation, bioassay, and ADC chemistry.
References
1. GlobalData Healthcare, October 5th 2023, ADCs dominate with billion-dollar licensing agreements in 2022 < https://www.pharmaceutical-technology.com/analyst-comment/adcs-huge-licensing-growth-continues/ >
2. GlobalData Healthcare, June 12th 2023, ASCO 2023 Highlights: Daiichi’s and AstraZeneca’s ADCs display impressive efficacy < https://www.clinicaltrialsarena.com/comment/asco-2023-daiichi-astrazeneca-adcs/ >
3. GlobalData, March 23rd 2023, Pfizer acquires ADC pioneer Seagen for $43bn < https://www.pharmaceutical-technology.com/comment/pfizer-acquires-adc-pioneer-seagen/ >
4. Seagen, n,d, Technologies, < https://www.seagen.com/science/technologies >
5. Gilead, April 7th 2023, FDA Approves Trolevy ® , the first the first treatment for metastatic triple-negative breast cancer shown to improve progression-free survival and overall survival < https://www.gilead.com/news-and-press/press-room/press-releases/2021/4/fda-approves-trodelvy-the-first-treatment-for-metastatic-triplenegative-breast-cancer-shown-to-improve-progressionfree-survival-and-overall-surviv >
6. Ambrx, October 23rd 2023, Ambrx Announces ARX517, a PSMA-targeted ADC, demonstrates a promising 52% PSA50 (≥50% reduction) and a highly differentiated safety and PK profile in patients mCRPC, who progressed on multiple FDA-approved treatments < https://ir.ambrx.com/news/news-details/2023/Ambrx-Announces-ARX517-a-PSMA-Targeted-ADC-Demonstrates-a-Promising-52-PSA50-50-Reduction-and-a-Highly-Differentiated-Safety-and-PK-Profile-in-Patients-with-mCRPC-who-Progressed-on-Multiple-FDA-Approved-Treatments/default.aspx >
7. ImmunoGen, November 14th 2022, ImmunoGen announces FDA accelerated approval of ELAHERE ™ (mirvetuximab soravtansine-gynx) for the Treatment of Platinum-Resistant Ovarian Cancer < https://investor.immunogen.com/news-releases/news-release-details/immunogen-announces-fda-accelerated-approval-elaheretm >
8. M. Vaidya, F. Barry, February 27th 2023, CMO Moves: Regulatory catalysts for drug manufacturing – February, https://www.pharmaceutical-technology.com/features/cmo-moves-regulatory-catalysts-for-drug-manufacturing-february/



