Cell cultivation is labour-intensive and includes many open process steps requiring expensive cleanroom procedures once the transition to clinical production is made. Consequently, there is an urgent need for efficient production platforms at scale.
Providing an innovative and cost-effective solution to this problem is the Bioscale project, a cross-border collaboration with partners from the Netherlands, Belgium, Switzerland, and Sweden. Bioscale aims to provide economically viable cell therapy using cultured human cells, by focusing on stem cell expansion technology.
“When you extract a biopsy from a patient you do not have sufficient cells for therapeutic purposes, so you need to scale them up. You need to multiply these cells and currently that is an intensive manual process,” explains Michiel Jannink, CEO of Netherlands based Scinus Cell Expansion, part of the Bioscale project. “You culture cells in flasks, and you repeat the process via multiple flasks until you have enough stem cells for your therapeutic purposes.”
Cultivating cells
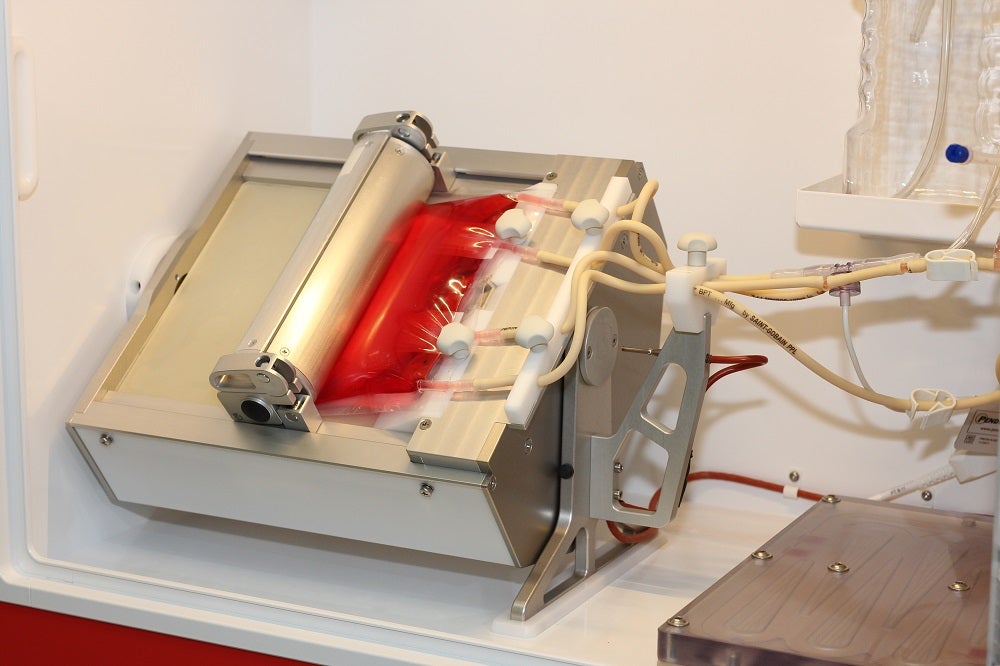
Developing this process means creating a system where cells can multiply and grow safely at scale. “That’s where we come in,” says Jannink. “Our bioreactor technology is an automized incubator system with a rocking platform, in which we can culture adherent as well as suspension cell types. Adherent cell types grow on a carrier structure, and we have a special single use bioreactor bag for that. In the bag we have included a filter with a pore size of about 100 microns, so when cells attach to the carriers, they have a combined diameter which is bigger than 100 microns and stay in the bag during the culture process.”
Cells consume oxygen during this cell culture process. With a perfusion loop system an optimal culture environment can be maintained for the cells within the bag. “The cells will stay within the bag because attached to the carriers they cannot pass through the membrane. Only the media will be pumped around, by which you keep that cell culture environment as optimal as possible for the cells.”
This process works well for adherent cells, but cells that grow in suspension need a different single use bioreactor bag working with the same Scinus Cell expansion system. One of the largest markets today is for CAR-T cell therapy, where modified T-cells are required. These cells are typically suspension cells, and do not grow on a carrier structure. “If we had that same bag,” notes Jannink, “T-cells – which are approximately 10 micron – will pass through those original filters and go into the perfusion loop, where they experience a lot of shear stress and will not survive. The adherent filter is not sufficient [fine enough] for T-cell culture, so we needed to create a different filter membrane.”
Developing a brand-new filter for T-cells
Scinus needed a completely new concept and turned to long-term collaborator, medical fabric specialist Sefar, for a different kind of filter solution, and together they developed an ultra-fine filter assembly of pore size 1 micron for a single use suspension bioreactor bag.
Jannink explains the concept behind the brand-new design: “We divide the bag into two compartments, the bottom compartment and a top compartment divided by the new filter membrane which has a pore size of about 1 micron. We grow the cells in the bottom compartment, with the perfusion loop on top. By this principle, the cells will stay in the bottom compartment because they cannot pass through that 1 micron filter membrane.
“On the other hand, crucial nutrients and oxygen can pass by means of diffusion through the filter assembly. That means that we can still feed the cells in the bottom compartment, give them enough oxygen, give them enough nutrients, and as such maintain an optimal culture environment for the cells.”
This new concept of a single use bioreactor bag means that Scinus can now address that large T-cell market.
Unrolling without deformation of the filter
Importantly, this new bag expands in size as the culture grows, explains Jannink: “We start culturing with a small amount of cells in a small culture volume, and as the cells start to grow, we can expand the bag by moving the roller [unrolling]. The filter, developed in conjunction with Sefar, is a three-layer filter assembly which not only has a small pore size of 1 micron but is also capable of being rolled up and rolled out, because that’s what we need.”
The Scinus patented bioreactor bag depends on the filter being extremely tough. It is essential that the filter doesn’t deform when rolled up, points out Jannink: “It needs to stay in shape because if we damage the filter and get bigger pore sizes, then those cells will pass into the perfusion loop, and they will die. That’s the innovation of the filter assembly.”
Expertise and consistent quality
Sefar’s experience was an essential part of the development of the new solution. Having worked with the filter specialist company before, Jannink says he already knew that Sefar products could be relied upon to be of consistently high quality: “You use these filters for medical applications – they need to fulfil certain requirements. Every time Sefar make a batch of new filter membrane assemblies, it needs to be done in a very controlled way. Each new batch needs to be the same as the previous.”
Sefar’s Marcel Rutz, the company’s global market manager for medical, comments that the Sefar team were keen to become involved in the project: “We have great cooperation with Scinus and with the whole team over there. For us it’s an interesting project because it’s about extending human health and that makes it very exciting to go into this field.”
Jannink concludes: “At Sefar, the quality and co-operation are good, and expertise is at a very high level. It’s important for us to have components in our bag which are well suited to culture cells and Sefar understands that. We work well together.”
To find out more, download the paper below.


