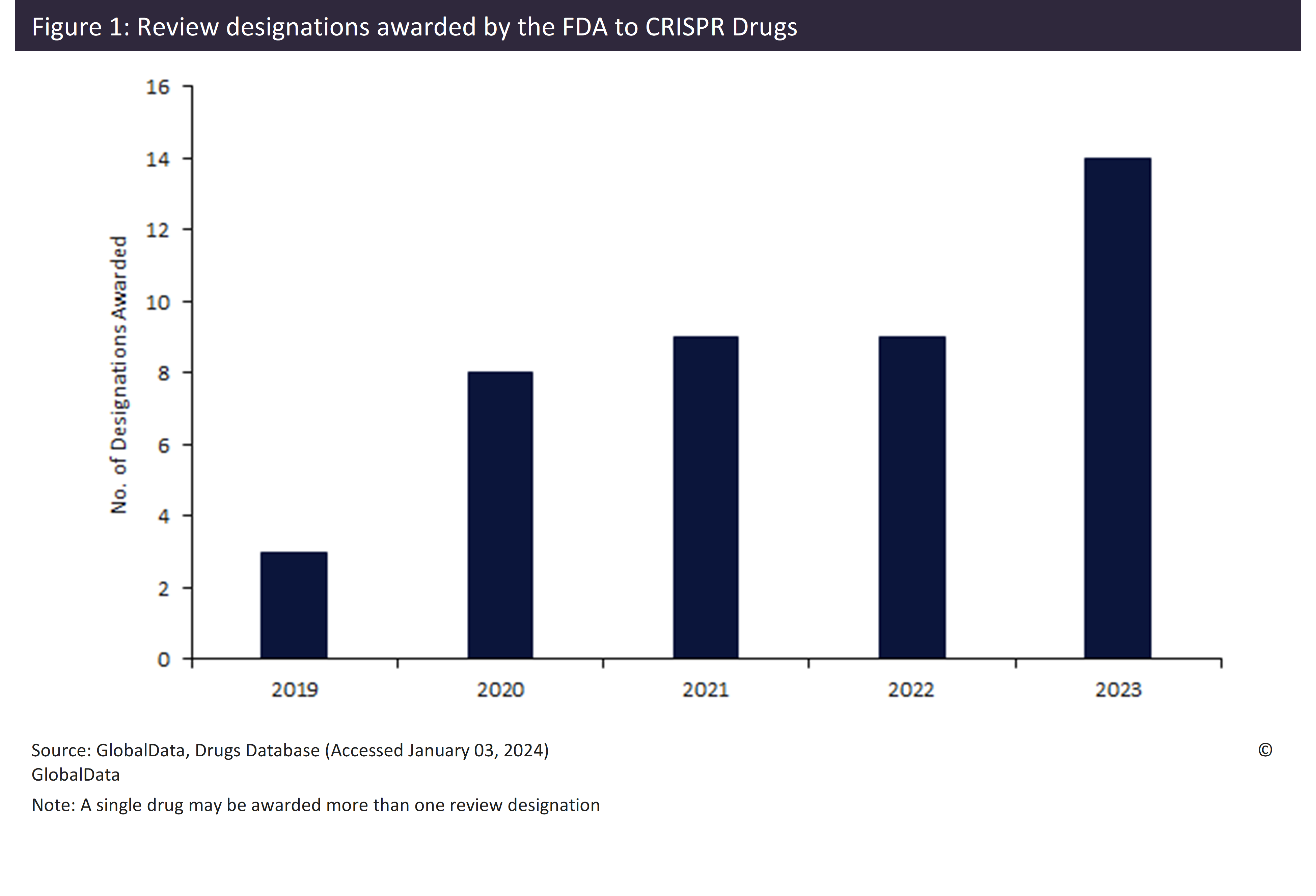
In late 2023, Vertex Pharmaceuticals and CRISPR Therapeutics made history by gaining the first FDA approval for a CRISPR-based drug, exagamglogene autotemcel (Casgevy). This historic achievement coincided with a record number of 14 review designations awarded by the FDA to CRISPR-based therapies in 2023. This increase in designations awarded in recent years may be part of an enhanced effort by the FDA to promote CRISPR drugs.
On 8 December, the FDA approved Casgevy for the treatment of sickle cell disease with vaso-occlusive crisis, becoming the first CRISPR-based drug to receive marketing authorisation globally.
CRISPR technology rectifies non-functional genes, as opposed to replacing or disrupting the pathogenic genes. Casgevy precisely edits the faulty gene in a patient’s bone marrow stem cell, enabling the production of functional haemoglobin. This replaces the previous permanent treatment option of a bone marrow transplant, which carries many risks, including rejection.
The 2023 approval of Casgevy occurred at the same time as the FDA awarded a record number of 14 review designations to CRISPR-based drugs. This record year saw six orphan drug designations, four fast track designations, two regenerative medicine advanced therapy (RMAT) designations, and a single rare pediatric disease designation and priority review, awarded to ten different CRISPR drugs.
The FDA awarded the first review designation to a CRISPR drug in 2019. Since this initial designation, the trend has been on an upward trajectory. The record 14 review designations in 2023 were a 55% increase from the previous high of nine, which occurred in both 2021 and 2022 (Figure 1, above).
Since 2019, the FDA has awarded 43 total review designations to 21 distinct CRISPR treatments. Over this period, the orphan drug designation has been awarded 22 times, and the fast-track designation, ten. The newly approved Casgevy stands out as the leader, having secured seven designations.

US Tariffs are shifting - will you react or anticipate?
Don’t let policy changes catch you off guard. Stay proactive with real-time data and expert analysis.
By GlobalDataFollowing Casgevy, Caribou Bioscience’s CB-011—a CRISPR-edited allogeneic CAR T-Cell therapy—emerged as a frontrunner in 2023, earning four review designations: two orphan drug designations and two fast track designations. One of each was awarded for refractory multiple myeloma and relapsed multiple myeloma.
The increasing number of review designations being awarded to CRISPR therapeutics, such as fast track designation, which accelerates the development and evaluation of drugs, suggests that the FDA understands the potential positive impact of these drugs. As such, the FDA is leading a concerted effort to support and promote the development of these drugs, which may result in another CRISPR therapeutic reaching the market sooner than initially anticipated.