For a disease group that cannot yet be cured, the administrations of therapies that slow the progression of the disease are imperative in prolonging the sufferers’ lives. In lysosomal storage disorders (LSDs), key opinion leaders (KOLs) interviewed by GlobalData are in agreement that a multitude of other ailments shroud various symptoms that help identify the disease group, allowing thousands of cases to be overlooked and undiagnosed every year. Taking action to combat this, market leader Sanofi Genzyme has teamed up with PerkinElmer Genomics to launch a comprehensive but free DNA testing program, the Lantern Project, raising awareness for the rare disease group while simultaneously expanding an incredibly niche drug market.
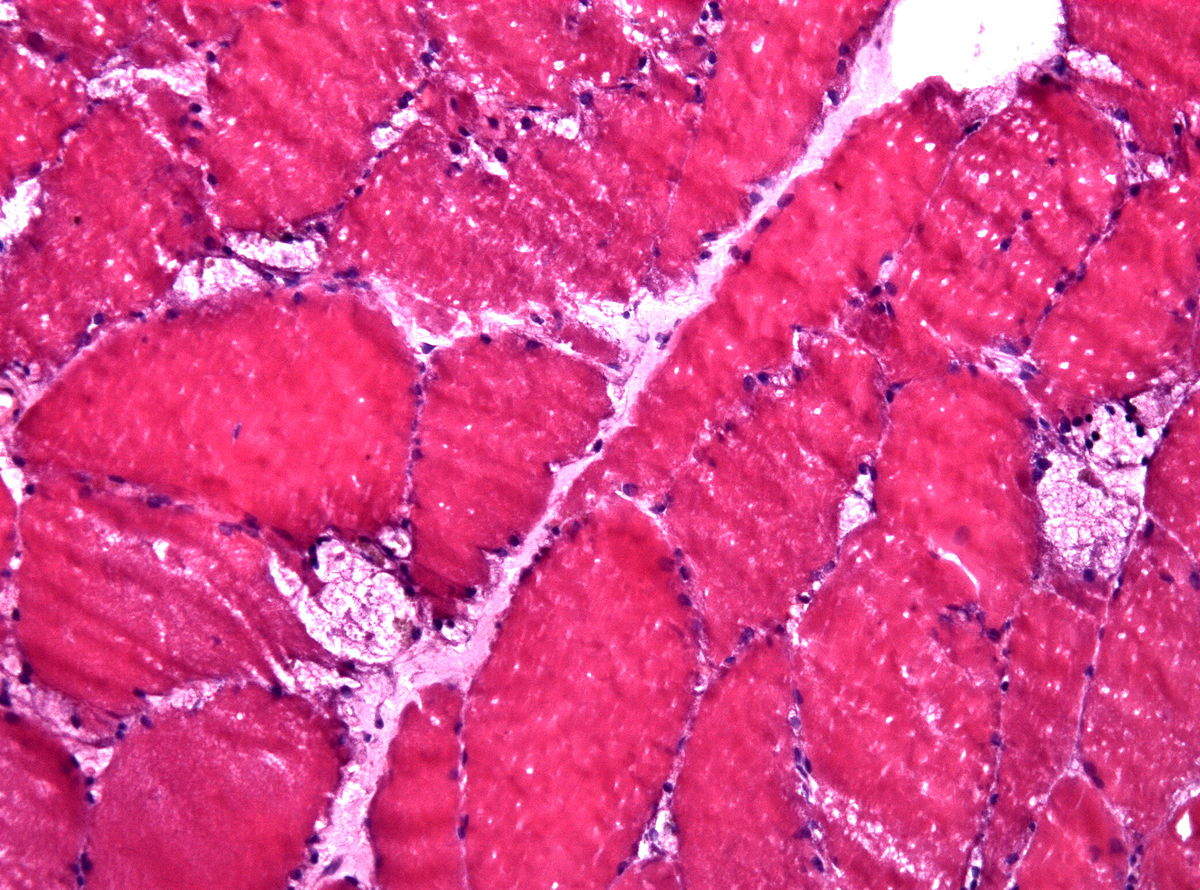
LSDs are inherited diseases resulting in the impairment or deficiency of enzymes crucial in governing metabolic processes: specifically, the breakdown of large molecules within lysosomes such as glycogen or lipids. These waste molecules accumulate, eventually leading to symptoms arising from progressive tissue and organ damage. To exemplify, if the treatment of late-onset Pompe disease (lysosomal glycogen accumulation from glucosidase deficiency) was conducted from birth, it would have the potential to significantly increase life expectancy, as well as allowing for a better quality of life in sufferers who would usually not receive their first dose until the intermediate progression of the disease. Furthermore, a larger patient pool has the potential to attract more interest in LSDs, theoretically increasing the developmental landscape of alternative therapies through company competition. This is crucial for Pompe disease sufferers, as the single standard enzyme replacement therapy from Genzyme leaves this disease with many unmet needs that won’t be addressed without a different approach.

US Tariffs are shifting - will you react or anticipate?
Don’t let policy changes catch you off guard. Stay proactive with real-time data and expert analysis.
By GlobalDataThe Lantern Project will begin with screening for Pompe, Gaucher, and Fabry diseases in the US, with additional enzyme panels for several mucopolysaccharidoses and other related myopathies. A better diagnosis benefits not only patients but companies marketing therapeutics for LSDs. This is due to far higher sales revenue being exhibited, which will make up for initial testing costs. Patients suffering from LSDs will have a significantly longer life, which may bridge the gap until a more auspicious treatment such as gene therapy can be developed.