Aptar Pharma Gains Approval by European Medicines Agency for Electronic Lockout Device
Aptar Pharma has announced approval by the European Medicines Agency (EMA) for integrated electronic nasal lockout device e-Lockout following a multi-year development with Takeda Pharmaceuticals International.
Aptar Pharma has agreed to supply Takeda with this new device for a multidose nasal spray version of Instanyl®. The EMA has granted marketing authorisation for this spray treatment under the name Instanyl DoseGuardTM.
Already available in unidose and multidose nasal spray versions, Takeda will launch Instanyl® DoseGuard throughout Europe in several multidose strengths. Each dose uses Aptar Pharma’s patented lockout system, which marks another product innovation in the management of breakthrough pain.
Advanced e-device technology ensures safe compliance
Instanyl® is a fast-acting nasal opioid approved for relieving breakthrough pain in adult cancer patients who are already being treated with opioids. Breakthrough pain is a sudden pain that occurs despite having taken pain relieving medicines.
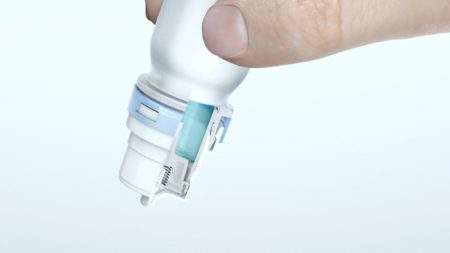
The system’s built-in lock-out mechanism prevents the device from being used for a period of time after a pre-defined number of spray actuations. The electronic display shows the number of priming strokes, the number of doses left in the device, and whether the nasal spray is locked or ready for use. The e-Lockout also features a child-resistant cap.
Long-term strategic partnership with Takeda
The multi-year supply agreement reinforces a long-standing partnership between Takeda and Aptar Pharma, which currently supplies Takeda with unidose and multidose nasal spray devices for Instanyl® in Europe.
Committed to accompanying pharmaceutical companies throughout their product lifecycle management, Aptar Pharma continues to partner to provide customers with innovative and smart solutions to enable safe, convenient and compliant medication delivery.
President of Aptar Pharma Salim Haffar said: "This approval and subsequent product launch underscores Aptar Pharma’s ability to partner with the pharmaceutical industry to bring innovative, compliant, and safer devices through the regulatory authorisation process.
"This is yet another example of Aptar Pharma’s expertise and technology at the heart of a new market launch. This is a significant step in strengthening Aptar Pharma’s credentials in the electronics and connected health markets. We are pleased to be building on our trusted, long-term partnership with Takeda."