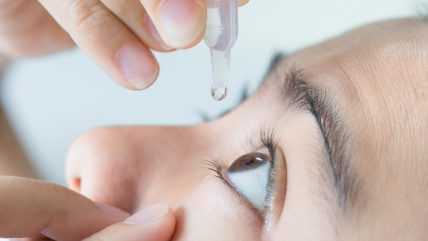
French ophthalmology company Nicox has licensed its IOP eye drop NCX 470 to Japanese pharmaceutical Kowa Company.
Kowa will gain exclusive research, development, and marketing rights for NCX 470 in Japan. The company will pay €3m ($3.2m) in non-refundable upfront payment to Nicox. The French company will also be in line to receive up to €10m in development and regulatory milestones, €17.5m in sales milestones along with 7–12% in tiered royalties on net sales, as per an 8 February press release.

Discover B2B Marketing That Performs
Combine business intelligence and editorial excellence to reach engaged professionals across 36 leading media platforms.
The company expects the funds from the agreement to extend its financial runway until September. Nicox had financial debts of €21m, as of 31 December 2023, with the company exploring debt restructuring and NCX 470 licensing to raise capital. To that end, Nicox is also looking for a commercial partnership in the US.
NCX 470 is a novel nitric oxide-donating bimatoprost being developed for lowering intraocular pressure (IOP) in patients with open-angle glaucoma or ocular hypertension.
In addition to the Phase III data gathered in a US-based trial, Kowa expects to conduct additional clinical trials with Japanese patients to obtain regulatory approval in Japan. The company will also bear all development, regulatory and commercialisation costs for NCX 470 in that country.
The drug was evaluated in a Phase III trial (NCT04445519), with an active comparator latanoprost, a prostaglandin analogue medicine marketed to treat increased IOP. The study showed that a 0.1% concentration of NCX 470 demonstrated a 1.73 mmHg greater mean diurnal IOP reduction from baseline, compared to latanoprost.

US Tariffs are shifting - will you react or anticipate?
Don’t let policy changes catch you off guard. Stay proactive with real-time data and expert analysis.
By GlobalDataThe second Phase III trial (NCT04630808) of NCX 470 is currently ongoing in patients with ocular hypertension or open-angle glaucoma, with results expected in 2025. Nicox expects the two-phase III data to be enough to support regulatory submission in the US and China.
Ocumension Therapeutics has developmental and commercial rights to NCX 470 in mainland China, Hong Kong, Macau, Taiwan, Korea and South-East Asia. Ocumension paid €3m in upfront payment for rights in China, Hong Kong and Macau, with Nicox being eligible to receive up to €33.25 in milestone-based payment and 6%-12% in tiered royalties on sales, as per the 2018 agreement.
In 2020, the companies amended their agreement to include Korea and South-East Asia on the list of territories where Ocumension holds commercialisation rights to NCX 470.




