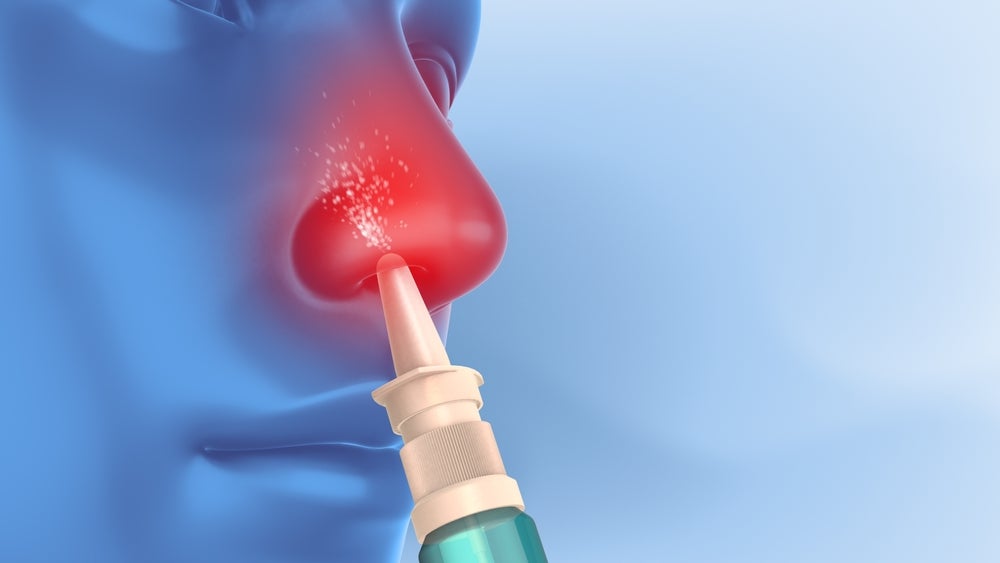
Pneumagen has raised £8m ($10 million) for the further development of its intranasal antiviral drug neumifil in Phase II studies for chronic obstructive pulmonary disease (COPD) patients suffering from virus-induced exacerbations.
A Phase IIb study of neumifil is currently planned to start in 2024, per the 1 June announcement. According to the company’s website, the Phase IIb is planned to begin in early 2024.

Discover B2B Marketing That Performs
Combine business intelligence and editorial excellence to reach engaged professionals across 36 leading media platforms.
The St. Andrews, UK-based company raised the funds from the venture investment company Esperante Ventures, alongside existing investors Thairm Bio and Scottish Enterprise. Pneumagen is originally a spinout from the Scottish University of St. Andrews.
Neumifil consists of an engineered carbohydrate-binding module domain of a Streptococcus pneumoniae protein. The vaccine is self-administered through an intranasal spray, and is based on the company’s GlycoTarge platform. The platform is derived from bacterial glycosidases and is researched for potential prophylactic use against respiratory viruses such as influenza. It can also be explored for other viruses like respiratory syncytial virus, human rhino virus and coronaviruses.
Additionally, the company expects to share initial results from the ongoing Phase IIa (NCT05507567) proof-of-concept challenge study in healthy subjects infected with influenza in mid-2023. The company dosed the first patient for this study in August 2022. The company had previously raised raised £3.8 million ($4.75 million) for the Phase II development of neumifil in January 2022.
In May 2022, Pneumagen shared positive topline data from a 60-participant Phase I study (NCT05093530). Neumifil was reported to be well tolerated with no dose-limiting toxicities and no serious adverse events.

US Tariffs are shifting - will you react or anticipate?
Don’t let policy changes catch you off guard. Stay proactive with real-time data and expert analysis.
By GlobalDataThe Scottish company previously studied neumifil and other multivalent carbohydrate binding modules against Covid-19. In April 2020, Pneumagen announced positive anti-viral activity results from three in vitro studies into preventing coronavirus infections, but the program has not yet been studied in humans.


