Chemical Characterisation


Considering the chemical characterisation of the materials that a device is made from is a necessary first step in assessing the biological safety of the device.
It is also important in judging equivalence of:
- A proposed material to a clinically established material, and
- A prototype device to a final device
The extent of chemical characterisation required should reflect the nature and duration of the clinical exposure and shall be determined by the toxicological risk assessor based on the data necessary to evaluate the biological safety of the device.
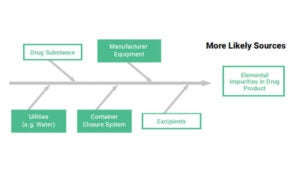
According to ISO 10993 – 1:2009, the following shall be considered for their relevance to the overall biological evaluation of the device:
- The materials of manufacture
- Intended additives, process contaminants and residues (see ISO 10993-7 for ethylene oxide residues)
- Leachable substances (see ISO 10993-17)
- Degradation products (see ISO 10993-9, for general principles and 10993-13, 10993-14 and 10993-15 for degradation products from polymers, ceramics and metals, respectively)
- Other components and their interactions in the final product
- The performance and characteristics of the final product
- Physical characteristics of the final product, including porosity, particle size, shape and surface morphology
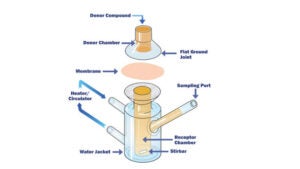
Chemical Characterisation comprises a variety of analytical techniques to identify and quantify materials that may have migrated from the product contact material into the solution of interest.
The purpose of this testing is to determine a baseline to the extractable amount of chemical compounds present in and on a medical device and utilise extraction conditions similar to those utilised in biocompatibility determination of similar devices.
QualiMetrix employs cutting-edge technology combined with sophisticated analytical knowledge and expertise, providing accurate and top-quality results. An indicative list of instrumentation used for chemical characterization is presented below:
- Gas chromatography-mass spectrometry (GC-MS), equipped with electron ionization (EI) source and head-space autosampler
- Liquid chromatography-mass spectrometry (LC-MS/MS), triple quadrupole mass (QqQ) equipped with electrospray (ESI) ionisation mode
- Liquid chromatography-high-resolution mass spectrometry (LC-HRMS/MS), hybrid linear ion trap (LTQ) Orbitrap system, equipped with heated electrospray (HESI) and Atmospheric pressure chemical (APCI) ionisation mode
- Inductively coupled plasma/mass spectrometry (ICP-MS)
In order to perform an adequate risk and safety evaluation for the compounds resulting from chemical characterisation studies, each compound’s structure should be elucidated to an extent that literature and structure-activity relationship assessment can be performed. Accurate mass measurements, together with distinct isotopic profile and fragmentation information can provide the means for structural elucidation and identification of possible leachable and extractable compounds.
A thorough toxicological risk assessment is performed on the notion that if all of the constituents of a medical device are known, then the safety of the device can be assessed based on the toxicology of those constituents. The methods for toxicological risk assessment are, in general, described in ISO 10993-17, ISO 18562, ICH M7 and Q3D guidance.