Track-and-Trace Platform
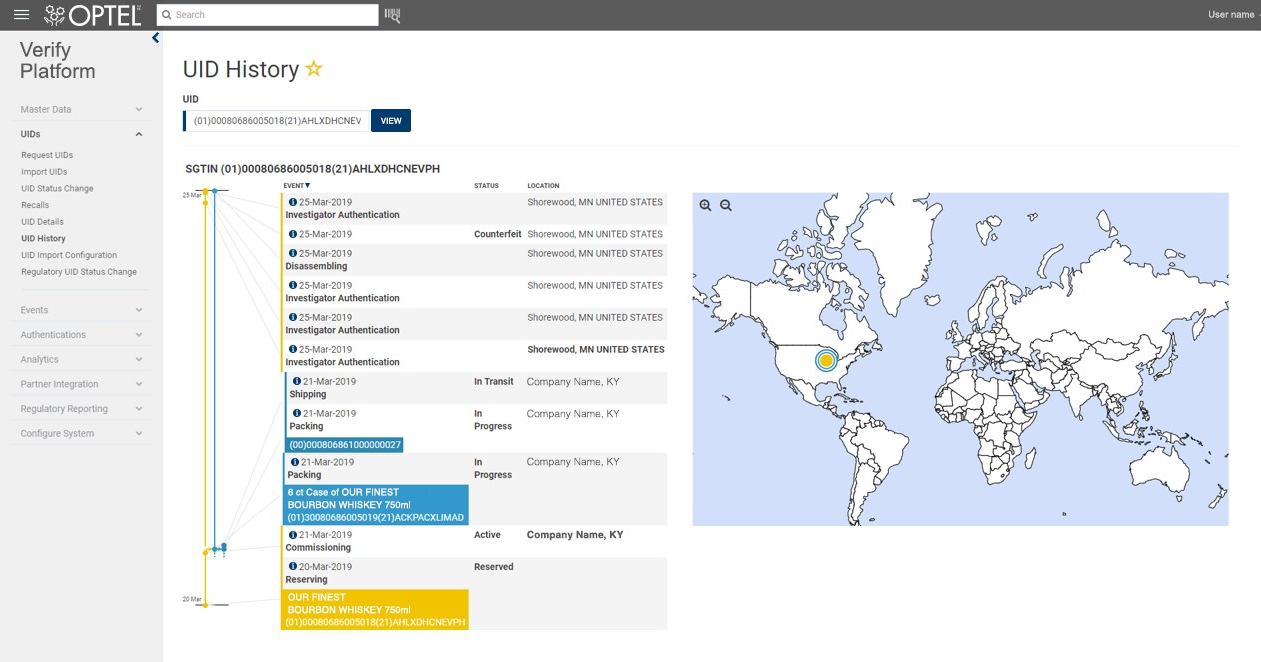

OPTEL’s true end-to-end digital traceability technologies allow you to view critical, granular data in a multidimensional context. Digital traceability enables authentication, safeguards against counterfeiting and grey-market diversion, and provides item-level traceability and chain-of-custody reports. OPTEL’s solutions ensure real-time visibility into your supply chain’s state of health, helping you track, trace, authenticate and ensure the quality and safety of your consumer goods, quickly identify sources of contamination or other issues, and manage targeted recalls faster and more efficiently.
Traceability can be defined as complete visibility over the product life cycle and supply chain. It’s like a unique fingerprint that identifies, verifies and follows individual items, such as medications, drugs, food and beverages, consumer packaged goods and even mines and minerals, from the raw materials to the consumer.
Through systematic, documented identification and recorded data, traceability allows you to verify and track the history, location, and even the state or status of an item, regardless of where it is in the supply chain.
As an integral part of its traceability platform, OPTEL offers reliable and flexible serialisation and aggregation solutions to help you comply with current regulations and adapt to all new and upcoming regulations, including tax stamps. Whether you are looking to implement serialisation in a matter of weeks with our turnkey solutions or are looking for a customised and adaptable solution for your unique challenges, OPTEL has a solution for you.
For the past three decades, OPTEL has developed and successfully implemented thousands of serialisation and aggregation solutions to ensure regulatory compliance and help pharmaceutical companies, CMOs and CPOs improve their quality assurance and increase the operational efficiency of their production and packaging lines.

