In June 2018, Pfizer announced plans to build a sterile injectables production facility at Portage in Kalamazoo County, Michigan.
The new sterile injectables production facility will be built with an estimated investment of $465m. It is part of Pfizer’s $5bn investment in US-based capital projects following the enactment of the Tax Cuts and Jobs Act, which reduced corporate tax to 10%.
Construction of the new facility is planned for mid-2019, with completion scheduled for 2021. The facility is expected to begin production in 2024, following validation by regulatory agencies.
The plant will allow Pfizer to manufacture critical injectable medicines for patients worldwide, as well as supporting the company’s production and supply capacity.
The project is expected to generate around 450 jobs in Portage. The governing body of the Portage County estimates that the facility will generate tax revenue of around $26m and have a regional impact of $49.2m over the next 15 years.
Pfizer’s sterile injectable production facility location
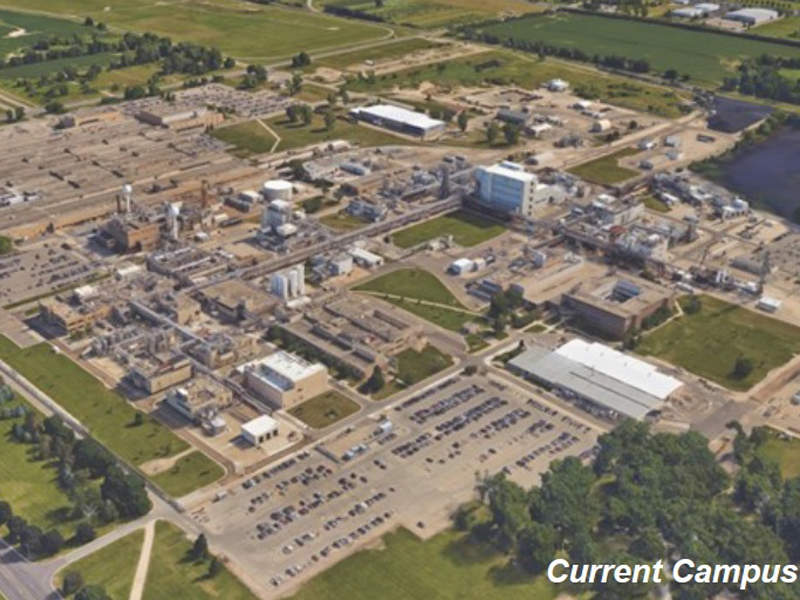
The facility will be developed on a site located within Pfizer’s existing Portage campus in Kalamazoo County.
Pfizer chose south-west Michigan as the ideal location for the facility due to the region’s favourable economy and manufacturing environment.
Facilities at Pfizer’s Portage production facility
The sterile injectable production facility will be a multi-storeyed facility covering a 400,000ft² area. It will feature state-of-the-art modular aseptic processing technology, equipment and systems.
The facility will be equipped with multiple sterile, self-contained mobile manufacturing lines to manufacture injectable drugs. Each of the facility’s processing modules will be entirely segregated from the remaining manufacturing lines.
The design will enable Pfizer to abide by the latest US Food and Drug Administration’s (FDA) directive to produce injectable drugs in sterile and self-contained production rooms.
The project is expected to require significant upgrades to the existing utilities and other infrastructure on the Portage site.
Financing and incentives for the Pfizer production facility
The Michigan Strategic Fund (MSF) is supporting the development by providing a $1m grant through the Michigan Business Development Programme, an incentive programme that creates qualified jobs.
Portage City Council will provide the company with industrial facilities tax exemption credits, which reduce property tax for 15 years.
Pfizer will also receive withholding tax capture valued at $10.5m for around ten years under the Good Jobs for Michigan programme, an incentive programme that aims to attract large-scale projects to the state. Of the 450 jobs expected to be generated by the facility, 354 will qualify under the programme.
Details of Pfizer’s Portage facility
The Pfizer Global Supply (PGS) campus in Kalamazoo County is used to produce sterile injectable drugs, liquids and semi-solid drugs, as well as active pharmaceutical ingredients (APIs).
Operating since 1948, the Portage site manufactures more than 150 products, including the injectable anti-inflammatory medicine Solu-Medrol.
The site employs more than 2,200 people and is estimated to have an economic impact of $2.2bn in the region.
The new sterile injectable manufacturing plant will allow the Portage site to compete globally. Pfizer is also planning to invest $1.1bn in Kalamazoo County over the next five years to increase its sterile manufacturing capabilities and meet the growing demand for its products.