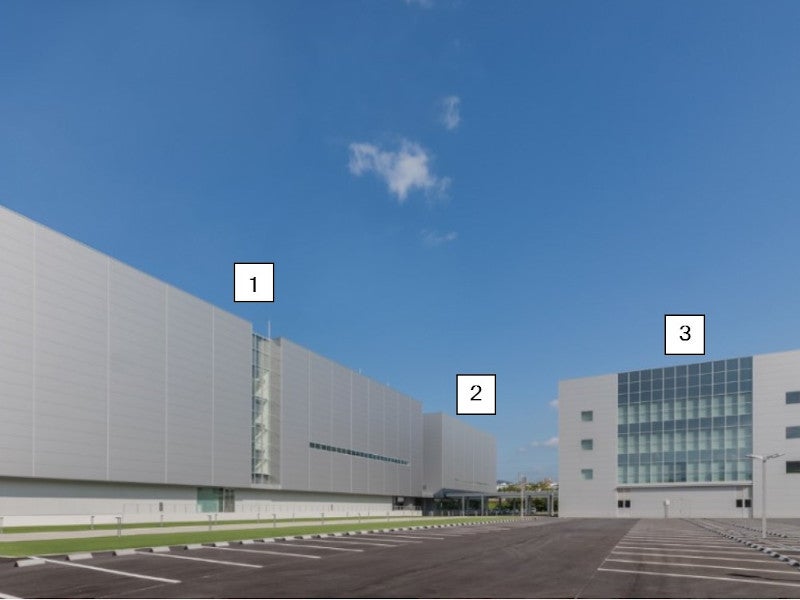
Takara Bio opened its new facility, the Center for Gene and Cell Therapy Processing II (CGCPII), in Shiga, Japan.
The facility will increase the capacity to offer more assistance to the company’s global client base in the production of regenerative medicinal products. It began operations in January 2020 and focuses on the development and production of regenerative therapeutic products.
Its location will allow Takara Bio to expand its CDMO activity and gene therapy initiative, targeting the development of new modalities of biological technologies, also taking over some of the functions of the company’s other existing facilities. Development of the facility and the expansion of capabilities at the headquarters involved the investment of JPY7.3bn ($67.05m).
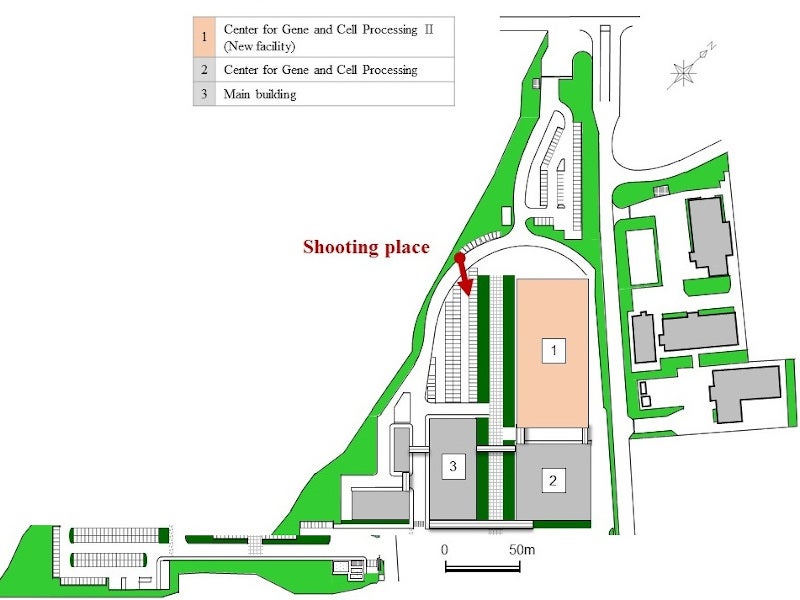
Location of Takara Bio’s Center for Gene and Cell Therapy Processing II
Center for Gene and Cell Therapy Processing II is located in Nojihigashi, Kusatsu, Shiga, West Japan.
The facility occupies a gross floor area of 14,500m² and is more than double the size of an existing facility at the location.
Approximately 4,600m² of the total floor space area is designed to cater to future needs and currently includes no manufacturing equipment.
Details of Takara Bio’s Center for Gene and Cell Therapy Processing II
The three-storey CGCPII building will expand the capacity of Takara Bio for viral vector production, process development, cell banking, cell processing, aseptic filling of pharmaceutical products and quality testing facilities, providing scope for further expansion in the future.
The facility is designed to support contract manufacturing services and research activities in areas including the production of regenerative medical products compliant with good manufacturing practice (GMP) and good gene, cellular and tissue-based products manufacturing practice (GCTP), quality testing and genome editing and manufacturing of iPS cells.
CGCPII is an extension of the company’s Center for Gene and Cell Processing (CGCP), built in 2014 and used to manufacture cell and gene therapy products.
CGCP centre was established to provide CDMO support for the development and production of regenerative medicines and to produce test drugs for the company’s gene therapy programmes.
Both facilities are accredited according to the standards of the International Organization of Standardization (ISO) 9001.
The manufacturing facility performs Good Manufacturing Practice (GMP) and General Cartographic Transformation Package (GCTP)-compliant production to meet the manufacturing control and quality control requirements for regenerative medicines and pharmaceuticals.
With the launch of CGCPII, Takara Bio can satisfy the increasing demand for gene and cell therapy products and will continue to develop its offerings for biopharmaceuticals and regenerative medicines under GMP and GCTP conditions.
The company’s manufacturing activities for gene and cell therapy products are conducted by experts and assist throughout the development life-cycle of regenerative pharmaceutical products.
The facility builds on the company’s successful development of clinical reagents such as RetroNectin® for research in regenerative medicines.
Marketing commentary on Takara Bio
Takara Bio specialises in biotechnology research and development, providing clients with a range of life science testing options, including enzymes, GMP reagents and licensed facilities for the manufacturing of cell and gene therapy products. The company originally started its operations as Takara Shuzo’s biomedical business unit. Its key business segments include the Bioindustry business and the Gene Therapy business.
Takara Bio is focused on leveraging biotechnology for disease prevention and enhancing the quality of life of people.
The company is also the developer of the RetroNectin reagent, used for retroviral transduction of genes in hematopoietic stem cells and other hard-to-infect cell types. The reagent is considered the global standard in gene therapy procedures.
Founded in April 2002, Takara Bio is headquartered in the Kusatsu town of Shiga Prefecture. The company has approximately 1,485 employees.