
According to GlobalData, the biopharmaceutical industry commenced 398 clinical trials for CAR-T therapies in 2024 – an increase of greater than 100 trials from previous years. 2025 looks set to be another record year, with 387 trials already commenced by mid-September.
But while research is ramping up, problems lie in the delivery systems. One issue is the limited infrastructure for leukapheresis, the critical first step in manufacturing an autologous CAR-T therapy.
Delivery bottlenecks
Only a small number of specialised treatment centres currently have the capabilities to perform leukapheresis, a lengthy procedure whereby blood is removed from the body, healthy white blood cells extracted for CAR-T manufacturing, and the remaining blood returned. Most of the time, such infrastructure is located within the prominent academic medical centres of large urban areas.
“Patients often travel long distances for leukapheresis or treatment, which is simply not feasible for many,” says Adrienne Mendoza, COO, BBG Advanced Therapies. “These geographic and socioeconomic burdens directly contribute to the lack of diversity in trial populations, and that affects both equity and data representativeness.”
Hospital capacity is becoming another issue, with many leukapheresis centres facing resourcing challenges, scheduling bottlenecks and competing priorities. As research in off-the-shelf CAR-T and Tumour Infiltrating Lymphocyte therapies continues to expand, this infrastructure will be further stretched.
One solution has the power to solve all of these challenges, but it requires the industry to reimagine a decades-old paradigm. Namely, instead of making patients travel to a leukapheresis centre, could the centre travel to them?
BBG Advanced Therapies, located in San Antoino, Texas, is currently launching a solution that does just that. The non-profit organisation is on a mission to advance patient access to cell therapies by supporting the industry with collection, testing, and biomanufacturing services, and has been performing peripheral blood stem cell collection and leukapheresis since 2009. This year, the company announced it had designed and built the world’s first mobile leukapheresis center, which will support immune cell collections across more than 63,000 square miles, expanding access for communities in a model that has never before been seen.
Designing and validating a mobile leukapheresis platform
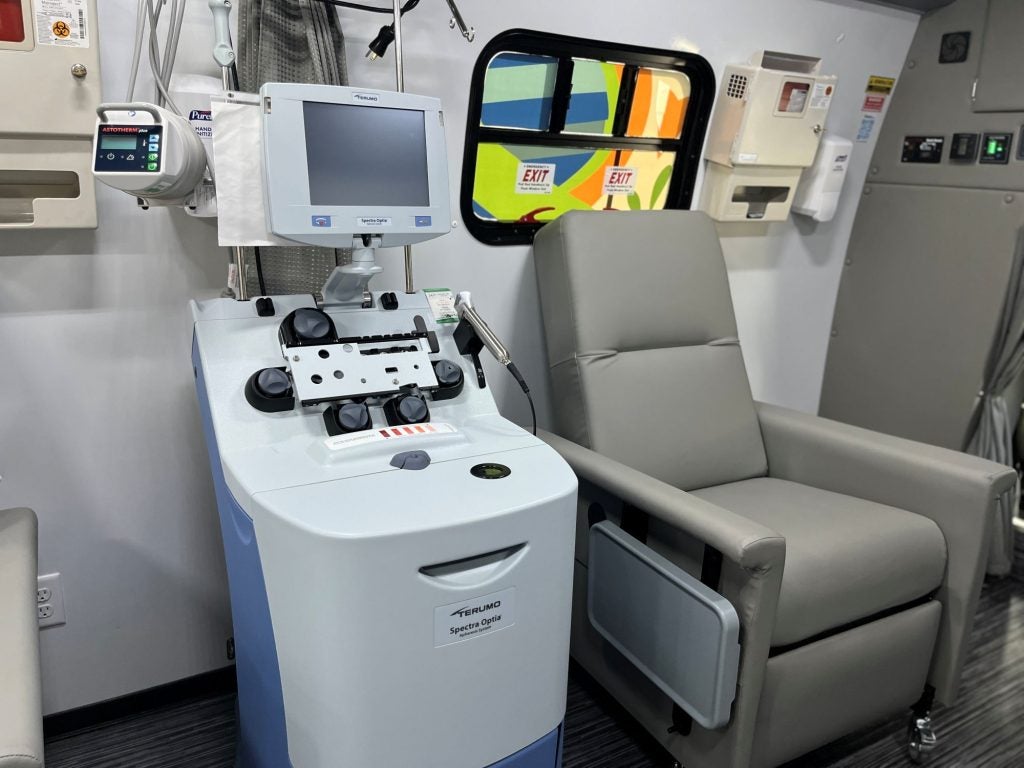
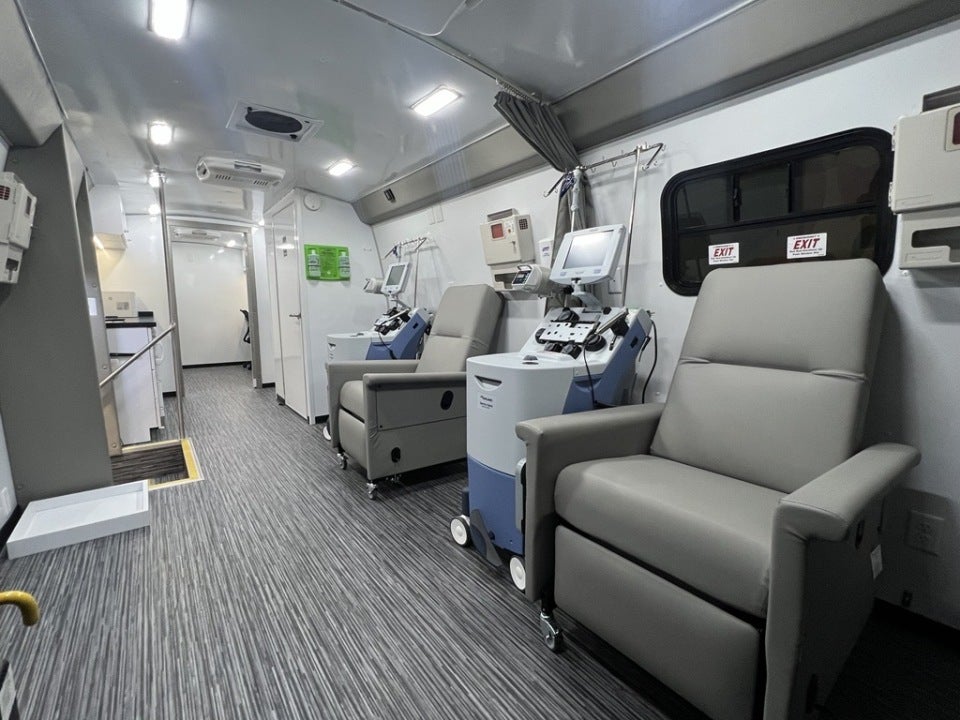
Vivienne Marshall, Executive Director of Starting Materials and Clinical Services for BBG Advanced Therapies, was heavily involved in the design process for the mobile leukapheresis center. She says the goal was to mirror the levels of control, quality, and oversight at BBG Advanced Therapies’ existing FACT-accredited leukapheresis centre, all while working within the space constraints of a bus-sized vehicle.
“For the design itself, that includes the ability to maintain appropriate temperature, and as part of that we have redundant power supplies on board as well as access to shore power, so the bus will be hooked up and running 24/7 just as you would expect at a fixed site,” she explains. “We will also have real-time environmental monitoring and the same collection and analysis equipment as our headquarters location.”
Meanwhile, all staff training requirements and standard operating procedures adhere to the quality management system used across the BioBridge Global organisation. This includes an electronic documentation system to support data integrity, traceability, and oversight.
The Foundation for the Accreditation of Cellular Therapy (FACT) has a process for adding the mobile facility to the existing accreditation for BBG Advanced Therapies, and the team has also received positive feedback from the Food and Drug Administration confirming that the unit will operate under the same regulatory paradigm as the company’s San Antonio fixed site. The upshot is that no additional regulatory registrations will be required compared to fixed sites, on behalf of sponsor companies using the mobile leukapheresis center in their clinical trials or commercial supply chains.
Earlier this year, biopharma delegates were welcomed onboard during two cell and gene therapy conferences in New Orleans, Louisiana. “A lot of the time, one of the first things that we heard was that it feels just like walking into a traditional apheresis center,” notes Marshall. “It’s roomier than it looks.”
She adds that many industry professionals shared the vision behind the mobile unit, which is to improve access to cell therapies, particularly among underserved communities.



Onboard operations
Naomi Herrera is the Senior Manager of Clinical Leukapheresis Services at BBG Advanced Therapies and will be responsible for heading up the mobile collections.
“Leukapheresis can take anywhere from three to six hours, depending on the patient’s condition and client specifications. For some collections, if we don’t get enough cells in one day it could turn into a two-day procedure,” she notes.
Outlining the process that will take place during mobile collections, Herrera explains there will be a pre-procedural screening, physical examination step to determine the patient or donor’s eligibility and suitability to undergo leukapheresis in a mobile setting. At this stage, patients are informed about the procedure, consent is signed, and a venous assessment is made to determine whether peripheral or central lines should be used. The highly trained nursing team closely monitors the collection procedure and ensures the comfort of the donors/patients throughout the process. Post-procedure, the nursing team ensures patients/donors are as comfortable as possible and can leave the facility in a safe manner.
Once the cells are collected, Herrera explains, they are analysed with an onboard Sysmex™ cell counter, and future plans include expansion of analytical capabilities on board. The leukopaks are packaged using validated shippers, ensuring all labelling and documentation requirements are met. Depending on proximity to the leukopak’s next destination, a courier may meet the team on-site for onward logistics, or the team will coordinate delivery to the appropriate location.
“During this whole time, from when our patient or donor is scheduled, during the screening process, the eligibility, the physical exam, and the collection, our quality assurance review is being done step by step,” she adds. This includes a sign-off step whereby a quality assurance professional reviews all documentation and ensures that everything meets specification before the collection leaves the mobile centre.
Scaling out leukapheresis
Mobile leukapheresis could be a game changer for an industry in need of scaling out rather than up. It also represents a more convenient option for patients who often live hours away from the nearest fixed site leukapheresis center. Recognising this need, the team at BBG Advanced Therapies is focused on finding ways to increase the mobile unit’s efficiency, boosting the numbers of collections it can deliver.
“We want to increase utilisation in a single location or region to minimise that travel downtime,” notes James Johnson, Director of Starting Material and Research Operations. “Accomplishing that is going to require a lot of active communication between all parties.”
Robust information about forecasts and expectations will be critical in determining where the bus will travel. This will be followed by alignment with patient scheduling coordinators, manufacturers, and clinical trial teams to ensure the unit availability lines up with patient or donor readiness, post-collection logistics, and downstream manufacturing capacity.
When it comes to site selection, Johnson says “the primary goal is to minimise patient travel”, but this requires careful balance against proximity to emergency care, travel time, cold chain logistics, and feasibility for an overnight set-up, since collections may start from 7am. Beyond these considerations, the mobile leukapheresis center can go where the need lies.
It’s time for a new era in patient access, one that is not limited by geography, patients’ realities, and over-burdened academic centres. By introducing the world’s first mobile platform for leukapheresis, BBG Advanced Therapies hopes to share a blueprint with the industry, showing how providing infrastructure locally is the key to widespread, equitable access.



