Elemental Impurities According to Q3D Guideline

Elemental impurities are traces of metals that can be present in finished pharmaceutical products and may be found from several sources.
Their levels should be controlled within acceptable limits, while ICH Q3D guideline (the guideline for elemental impurities) has established permitted daily exposure (PDE) thresholds for each element of toxicological concern.
In line with the ICH Q3D guideline, QualiMetriX provides a full package of services regarding elemental impurities including risk assessment, testing of the final products and active pharmaceutical ingredients (API) and validation of the corresponding methodologies.
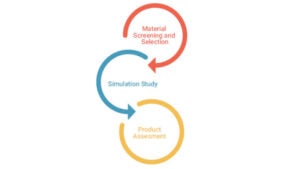
The first step of the risk assessment is to identify the risk factors to be considered:
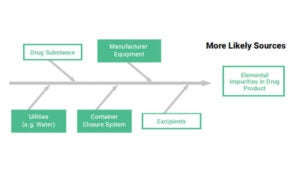
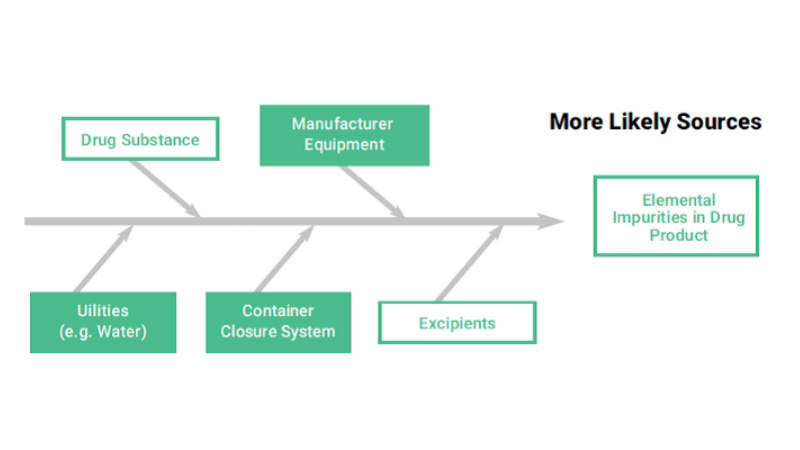
• API, manufacturing equipment, utilities, container closure system, and excipients that constitute the potential sources of elemental impurities in the final product
• Composition of the dosage form, API, excipient and drug product manufacturing process are reviewed in order to identify known and potential sources of elemental impurities. The elements that have to be considered are Class 1 and 2A metals, 2B and class 3, depending on the route of administration.
During the next step, the presence of particular elemental impurities in the drug product is being evaluated by gathering data from the scientific literature, data generated from similar processes, supplier information data and testing of the components of the drug product or the final product itself.
A combination of testing and risk-assessment is implemented by the lab in order to provide a comprehensive report.
Inductively coupled plasma mass spectrometry (ICP-MS) of the latest technology is employed in the laboratory.
Method validation according to USP <233> is performed, or even reduced schemes as method verification and sample analysis for all metals.