Changing tastes: Sofgen Pharmaceuticals prepares for increasing demand for softgels by expanding production capacity
Easy to swallow, appealing to the eye, and formulated to avoid any unpleasant aftertaste of what lies inside, it is…

Sofgen Pharmaceuticals is a leading provider of contract development and manufacturing services in softgel advanced technologies for the global pharmaceutical and nutraceutical industry.
You have successfully submitted your enquiry. Someone from our company will respond ASAP

Sofgen Pharmaceuticals is an integrated Softgel CMO/CDMO specialising in comprehensive solutions for softgel technologies. We support our partners across the entire lifecycle from development to delivery focusing on generics, branded products in prescription drugs, OTC, and specialised nutraceuticals for markets in North America, Canada, and Europe.
With two state-of-the-art softgel facilities, certified by the US FDA, TGA, Health Canada and UK MHRA among others, and R&D centres in the US and Colombia, we excel in high-end softgel manufacturing, including complex oral modalities such as hormonal products, high-potency compounds, and differentiated delivery technologies.
Our diverse service portfolio spans:
With a comprehensive range of CDMO services in diverse technologies, designed to elevate any pharmaceutical projects, we are focused on supporting customer needs and exceeding their expectations.
Our purpose is to provide an unparalleled experience with reliable development manufacturing services and niche proprietary products, innovation in technologies, contributing to the health and wellbeing of patients and consumers and in key markets.
At Sofgen Pharmaceuticals, we offer innovative and advanced softgel technologies for RX, OTC, and specialised nutraceuticals including:
At Sofgen Pharmaceuticals, we work with innovation, quality, and flexibility to develop and unleash the power of your most challenging projects. With an in-depth knowledge on development services, our experts are driven by science and technology to support all your projects along the way.
Our R&D services include:

Easy to swallow, appealing to the eye, and formulated to avoid any unpleasant aftertaste of what lies inside, it is…

Sofgen, a supplier of softgel technologies for generics, prescription drugs, OTC products and specialised nutraceuticals, will be exhibiting at the upcoming CPHI Europe conference in Milan, Italy. Taking place from 8-10 October, the conference will bring together 2,400 exhibiting companies from more than 160 countries, hosting over 100 content sessions led by more than 150 expert international speakers.

Sofgen Pharmaceuticals is an integrated CDMO/CMO that offers end-to-end from development throughout delivery for prescription drugs, OTC products and specialized nutraceuticals in Softgel advanced technologies.

Sofgen Pharmaceuticals, a leading provider of development and contract manufacturing services in advanced Softgel technologies for the pharmaceutical and nutraceutical industries, is proud to announce the unveiling of its new website, with the identity of a new corporate brand that promotes the innovation and transformation of the company.

Health Canada certifies Sofgen Pharmaceuticals in Canadian Good Manufacturing Practices (GMP) for our compliance with the Food and Drug Regulations at its facilities. This new permit allows our production areas to manufacture, package, label, distribute, import, wholesale, and/or test a drug.

The Softgel capsule has been on the market since the 19th century, evolving to the present day. Because of this, consumers feel familiar with the technology, trusting in its efficiency, robust quality control and convenience.

First-to-market technology ideal for organisations who want their consumers to have simplicity and effectiveness backed by technology and expertise when taking their meds and supplements.

Discover our Chewable Softgel Platform Chewgels™, chewable soft capsules as a solution and line extension to existing or new products. With new ingredients, special shapes and flavours, we can offer our customers a range of formulations for different therapeutic lines.
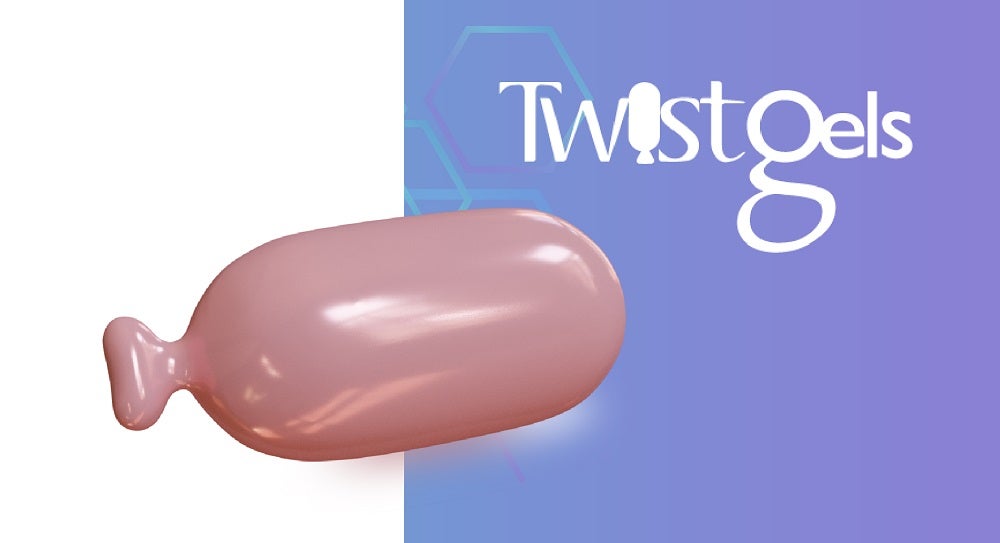
A soft gelatin capsule with twist-off cap that allows access to a measured dose for topical and/or oral applications.
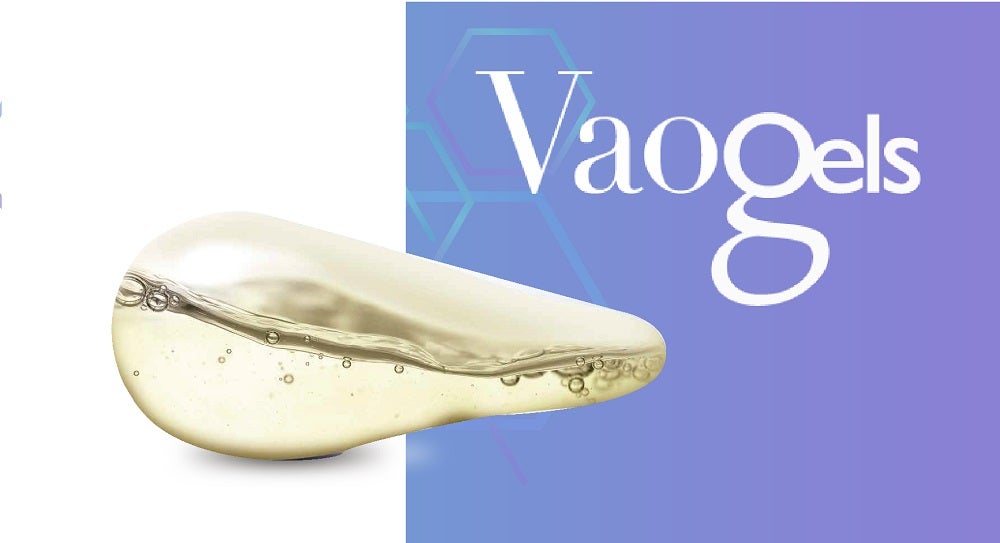
The softgel vaginal ovule or insert, Vaogels, was designed with mucoadhesive properties to facilitate retention of the product in the vagina and availability of the active ingredient in a site-specific manner.

With Reducedgels, consumers receive full strength and power in a smaller size softgel.