Considerations and Options for Prefilled Syringes
A prefilled syringe is a convenient primary packaging option for delivery of parenteral medication.

Baxter's contract manufacturing organisation (CMO) BioPharma Solutions offers form / fill / finish services for injectables.
You have successfully submitted your enquiry. Someone from our company will respond ASAP

Baxter’s contract manufacturing organisation (CMO) BioPharma Solutions offers form / fill / finish services for injectables.
With a successful combination of strong collaborations and more than 85 years of injectable drug experience, the company provides manufacturing services for a wide range of pharmaceutical drugs, including for the areas of oncology, biologics, antibody-drug conjugates (ADC), small molecules and vaccines. These are applicable for a variety of sterile dosage forms, including pre-filled syringes, cartridges, diluents for reconstitution, prefilled syringes, and lyophilised / liquid vials.
Baxter BioPharma Solutions helps clients with challenges in clinical supply, formulation, risk mitigation, patent expiry, scale-up of production, and addresses rises and falls in market demand.
Each solution is tailored specifically for each individual case, meeting clinical, commercial, traditional, and complex issues in sterile manufacturing.
With more than 50 manufacturing facilities worldwide, Baxter BioPharma’s award-winning current good manufacturing practice (cGMP) production facilities provide unique, high-value collaboration opportunities. The company’s extensive global network utilizes a key systemic approach to cGMP manufacturing to share efficiencies and expertise.
Baxter BioPharma Solutions’ manufacturing units have recently won 2016 Facility of the Year (FOYA) award for Operational Excellence; 2012, 2013, 2015, 2016, 2017, 2018 and 2019 CMO Leadership Awards from Life Science Leader magazine; and the 2010, 2011, 2012, 2015 and 2017 Best Contract Manufacturing Organization at the Vaccine Industry Excellence Awards.
Based in Bloomington in Indiana, Baxter BioPharma Solutions’ state-of-the-art 600,000ft² campus is one of the largest contract manufacturers of sterile products in North America. This facility helps clients develop and manufacture quality products and packaging, with particular expertise in regulation and lyophilisation.
Baxter BioPharma Solutions’ facility in Halle in Germany focuses on the manufacturing of cytotoxic and highly potent drugs. With more than 60 years of experience, the facility is certified by Safebridge and provides clinical and commercial production, with integrated technologies and services.
The recently expanded facility is dedicated to contract manufacturing of oncology products, such as cytotoxic, highly potent compounds, and ADCs. It received the FOYA in 2016 for Operational Excellence. Capabilities include liquid and dry powder filling, sterile crystallisation, disperse systems, clinical and commercial-scale lyophilisers, and flexible compounding areas.
Helping tackle technical challenges such as technology transfer and formulation, process, and analytical development, Baxter BioPharma Solutions’ teams help clients develop freeze-fried dosage forms, solutions, and suspensions for full optimisation of drug products throughout manufacturing.
The company provides expertise in optimisation and development in lyophilisation, converting drugs from a lyophilised to a liquid state through reformulation, converting drugs from vial to prefilled syringe form, analysing extractables and leachables, and selecting components, including flexible containers, syringes, vials, and stoppers.
Baxter BioPharma Solutions’ scientific team provides a large amount of industry experience across a wide variety of drug categories, including vaccines (adjuvant, conjugate), biologics (monoclonal antibodies, therapeutic proteins), cytotoxics, highly potent compounds, and antibody-drug conjugates (ADC).
Increasing stability for injectables and improving lyophilisation cycle times are vital parts of parenteral product development. With this optimisation, Baxter BioPharma Solutions formed the Lyophilization Center of Excellence to act as a resource centre for the production of high-quality freeze-drying.
Scientists and educators Dr Steven Nail and Wendy Saffel-Clemmer lead Baxter’s development team to assist with the modification and reformulations that optimise lyophilised products. The company offers:

A prefilled syringe is a convenient primary packaging option for delivery of parenteral medication.

Manufacturing in multi-product facilities affords numerous advantages but also presents significant challenges.

The production of seasonal vaccines, such as those for influenza, presents unique challenges to manufacturers due to the necessary time constraints resulting from annual strain selection.

Simtra BioPharma Solutions will expand its sterile fill/finish manufacturing capabilities at its Bloomington site in Indiana.

In August 2016, German pharmaceutical company Fresenius Kabi announced plans for an expansion of the pharmaceutical manufacturing capabilities of its site in Melrose Park, Illinois.

In May 2017, Chinese animal vaccine company Jinyu Bio-technology unveiled plans to establish its first US-based vaccine research lab and office facility, located in Kansas.

US-based biopharmaceutical company Baxalta, a spin-off of Baxter International, began building a biologics facility in Georgia, US, in August 2012.

Investment will add a syringe filling line, additional liquid and lyophilized (freeze dried) vial capacity and expand manufacturing footprint

Baxter International, a global leader in sterile medication production and delivery, and Moderna, a biotechnology company pioneering messenger RNA (mRNA) therapeutics and vaccines, today announced that they have entered an agreement for Baxter BioPharma Solutions to provide fill/finish sterile manufacturing services and supply packaging for approximately 60-90 million doses of the Moderna Covid-19 Vaccine in 2021.

Baxter International has announced that Baxter BioPharma Solutions has entered into an agreement to provide sterile manufacturing services for NVX-CoV2373, Novavax’ Covid-19 recombinant nanoparticle vaccine candidate with Matrix-M™ adjuvant.

Global leader in sterile medication production and delivery, Baxter International, is pleased to announce a $50 million expansion of its sterile fill/finish manufacturing facilities in Bloomington, Ind.

Baxter International Inc representatives globally are rising to the call and making a difference in acknowledgement to Covid-19.

Baxter BioPharma Solutions is pleased to announce that it will be an exhibitor at CPhI Worldwide 2019 on 5-7 November in Frankfurt, Germany.

Baxter’s BioPharma Solutions facility in Bloomington, Indiana, was recognised for the fifth time as the Best Contract Manufacturing Organization at the annual Vaccine Industry Excellence (ViE) Awards, held during the 2017 World Vaccine Congress in Washington, DC.

Baxter's BioPharma Solutions business is pleased to announce that the company's oncology manufacturing facility expansion in Halle, Germany is a winner of the 2016 Facility of the Year Award (FOYA) in the category of Operational Excellence.

Baxter's BioPharma Solutions and Sigma-Aldrich Corporation's (SAFC) commercial custom manufacturing services business unit have established a collaborative manufacturing agreement.

Pharmaceutical / biopharmaceutical clients of the contract manufacturing organisation (CMO) will be able to leverage BPS's expanded Drug Master File and choose a custom-fill volume and labelling, while streamlining development work and process validation batches, eliminating capital equipment costs. This can considerably reduce time to market.

Parenteral cytotoxic agents are sensitive drugs to handle and produce.

BioPharma Solutions, a global contract manufacturing leader in prefilled syringe fill-finish, provides clinical through high-volume commercial sterile manufacturing.
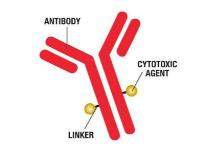
Baxter's BioPharma Solutions business (BPS) and SAFC (a business unit of Sigma Aldrich) are offering a combined approach for ADC requirements to simplify supply chain and provide comprehensive, coordinated and collaborative services for the development, manufacturing, and testing of bulk drug substance and drug product.

BioPharma Solutions' facility in Bloomington, Indiana, USA is one of the largest contract manufacturers of sterile products in North America, with a state-of-the-art 600,000ft² campus.

Optimising lyophilisation cycle times and improving stability for complex injectables is a critical component of parenteral product development.

An industry leader with a global presence, Biopharma Solutions is a contract manufacturing organisation (CMO) with a focus on injectable pharmaceuticals.

Cancer incidence is on the rise and cytotoxic therapies continue to be at the centre of oncology treatment programs. Through this latest expansion in Halle, Germany, Baxter BioPharma Solutions will continue to support pharmaceutical companies' effort