Pfizer's Biotechnology Campus, Clondalkin, Ireland
Pfizer will invest €1.2bn ($1.26bn) to expand its Biotechnology Campus in Grange Castle by 2027.

You have successfully submitted your enquiry. Someone from our company will respond ASAP
BTL provides specialist services, training and advanced instrumentation in the field of freeze drying.
Freeze drying (lyophilisation) has applications in the preservation of many different types of materials, including chemicals, proteins, bacteria, nano- and micro- particles, polymers, liposomes, parenteral and non-parenteral drug formulations, high-value food products and supplements and coatings.
Common reasons for freeze drying include extension of shelf life, management of reactive properties, preservation of biological activity, preservation of tissues and cells, and as a concentration step. By controlling ice formation during the freezing stage, freeze drying can also be used to create products with specific pore size, for example collagen scaffolds.
BTL believes that successful freeze drying formulations and cycles should be developed through rational, knowledge-based practice.
BTL’s freeze drying laboratory utilises a range of instruments and techniques to characterise products in terms of their freeze drying parameters in order to fully understand how they will behave before, during and after freeze drying. This ensures that cycles can be tailored to the specific needs of every product, making them efficient, safe, robust and reproducible.
The BTL team has decades of experience in the application of all aspects of freeze drying technology and has successfully processed over 700 different substances on behalf of clients. When taken together with our knowledge of pilot-scale and industrial freeze-dryers, we offer a uniquely comprehensive service covering all aspects of freeze drying from pre-formulation through to full-scale production and dried product analysis.
We aim to meet the precise needs of our customers’ projects, and will agree a work programme and a budget that is appropriate to the size and stage of your project. We are happy to run a single cycle or individual analysis for our clients, or agree a complete formulation and cycle development programme with you. Our philosophy is to augment your in-house expertise and work with you to make your project a success.
We offer a range of freeze drying services, as well as training, R&D and instrumentation. Our capabilities include:
BTL is keen to leverage its freeze drying process and engineering expertise in collaborative R&D projects. In the past two years, BTL has been awarded five grants by the UK government for feasibility studies and collaborative R&D projects focusing on applications such as the freeze-drying of blood, probiotics, collagen implants, herbicides and fusion proteins.

Pfizer will invest €1.2bn ($1.26bn) to expand its Biotechnology Campus in Grange Castle by 2027.

Baxter Biopharma Solutions is expanding its cytotoxic manufacturing facility in Halle in Westfalen, Germany. It is one
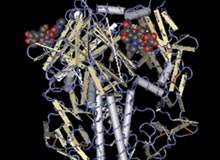
Sanofi Pasteur, the vaccines division of Sanofi Aventis, is focusing on a continuing technology upgrade and expansion. T
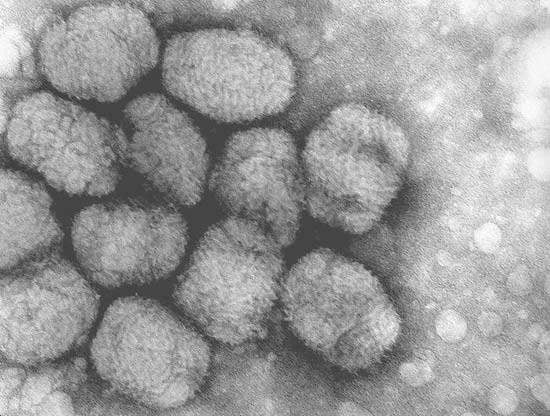
The Danish biotech company Bavarian Nordic has set up a new smallpox vaccine production facility in Kvistgård, Nort
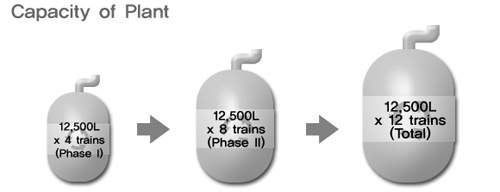
Celltrion, a joint venture between San Francisco-based Vaxgen and a group of South Korean investors, completed the const
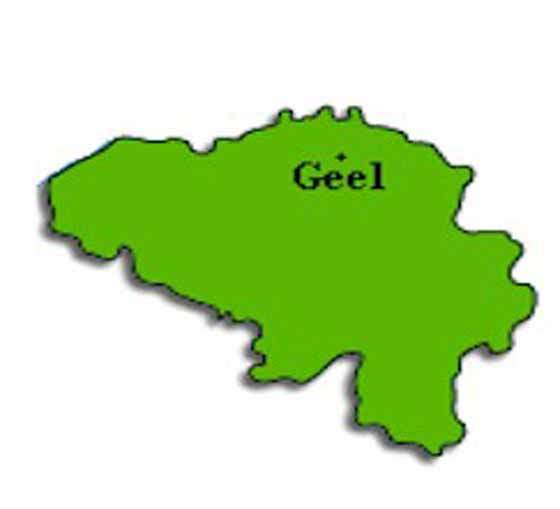
Johnson & Johnson (J&J) subsidiary Janssen Pharmaceutica decided to build a new pharmaceutical plant in Geel in Belgium in 1998.

Lyophilisation, or freeze drying, is an important process in many areas of research and production with applications in areas such as vaccines, diagnostics, regenerative medicine, functional foods, materials science and medical devices. For high-value products such as these it is vital to have a

BTL's freeze drying microscopes, the Lyostat series, have been popular for many years because of their speed and accuracy in returning useable data. Lyostat3, the latest in the series, features upgraded hardware and software and many options to offer enhanced reliability and flexibility, while ma

We are pleased to offer practical one-day 'refresher' sessions on the use of our freeze-drying microscopes, Lyostat3 and the previous model Lyostat2, and the Lyotherm2 thermal analyser. Lyostat and Lyotherm A rational, knowledge-based approach to designing and developin

Many medical diagnostic products are not stable in liquid formulation. Diagnostic agents are by definition prone to reaction and change at both ends of the molecular size range. The benefits of freeze drying As a stabilisation method freeze drying offers many benefits.

BTL is pleased to co-host this free half-day event, highlighting some of the key technological advances in freeze drying. This meeting will showcase presentations highlighting the latest developments from academia and industry perspectives on the new frontiers of freeze drying and the p

Lyophilization specialist BTL is pleased to welcome Dr Laura Ciccolini as its new head of sales. BTL is a renowned expert in freeze drying (lyophilization) technology with a range of consultancy services for development, optimization and scale-up of products and processes. Freeze dryin

Lyophilization specialist BTL is pleased to announce details of its training courses in lyophilization technology for 2011. Freeze drying is a complex science, requiring understanding and control of a number of different processes simultaneously. The difference between success and fai