In this last 2022 edition of the series, which started in June, Pharmaceutical Technology is tracking major trial announcements and decisions by regulators and reimbursement agencies that have occurred since mid-October, as well as their potential impact on manufacturing plans.
Pharmaceutical companies often outsource different parts of the manufacturing process, including the production of small molecule active pharmaceutical ingredients (APIs) and parenteral packaging, to contract manufacturing organizations (CMOs). The successful implementation of these contracts is necessary to ensure that a newly approved drug or an investigational therapy that is ready to advance to the next pivotal stage of clinical evaluation is manufactured in a timely manner.

Discover B2B Marketing That Performs
Combine business intelligence and editorial excellence to reach engaged professionals across 36 leading media platforms.
The data, which is collated from the GlobalData Pharma Intelligence Center’s Deals Database and PharmSource reports on these outsourcing relationships, provides a glimpse of not only the different players involved in the pharma supply value chain, but also the nature of the contracts that link them.
Regulatory decisions and clinical trial news
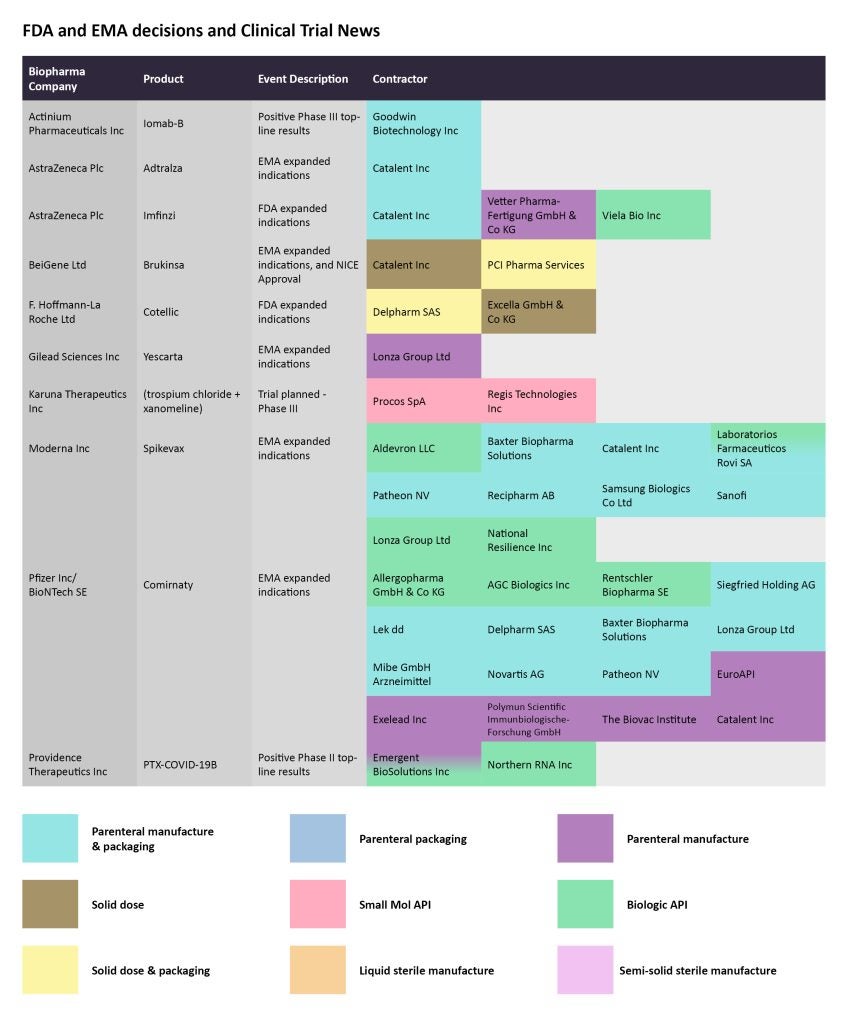
On October 31, Actinium Pharmaceuticals announced positive topline results from a Phase III study with Iomab-B, which is being studied to treat patients with active relapsed or refractory acute myeloid leukemia. Iomab-B met the durable complete remission endpoint. Previously, Iomab-B received an EMA orphan drug designation for patients with this condition. The parenteral manufacturing and packaging of this targeted radiotherapy has been assigned to Goodwin Biotechnology.
A few months after announcing positive results from one Phase III schizophrenia study in August, Karuna Therapeutics said it would initiate a Phase III program studying its drug KarXT (trospium chloride + xanomeline) as an adjunct therapy for schizophrenia. The Phase IIIb, six-week, outpatient study is to start in the current quarter, and Karuna will file an NDA for the drug in mid-2023, as per the company’s Q3 results. Procos SpA and Regis Technologies are contracted to produce the small molecule API.
Source: GlobalData Pharmaceutical Intelligence Center
Pfizer/BioNTech’s Comirnaty and Moderna’s Covid-19 vaccine products received another fillip when the EMA recommended the use of the vaccines targeting the original SARS-CoV-2 strain for children in specific age groups. The European regulator extended Comirnaty’s authorized use to those between the ages of six months and four years. Allegropharma GmbH & Co KG, AGC Biologics, and Rentschler Biopharma SE are tasked with producing the biological API for the vaccine. Several CMOs, including Siegfried Holding AG, Lek dd, Delpharm SAS, and Baxter Biopharma Solutions, are set for the parenteral manufacturing and packaging.

US Tariffs are shifting - will you react or anticipate?
Don’t let policy changes catch you off guard. Stay proactive with real-time data and expert analysis.
By GlobalDataThe EMA also recommended Moderna’s Spikevax for children ages six months to five years. Aldevron LLC, Lonza Group Ltd, National Resilience Inc, and Laboratorios Farmaceuticos Rovi SA are manufacturing the biologic API for the vaccine.
Regulatory and reimbursement decisions in the UK
In the UK, the Medicines and Healthcare products Regulatory Agency (MHRA) determines which medicines and medical devices as safe to use in the UK. The National Institute for Health and Care Excellence (NICE) is the authority that recommends the use of therapies based on a cost effectiveness analysis for the National Health Service system.
Source: GlobalData Pharmaceutical Intelligence Center
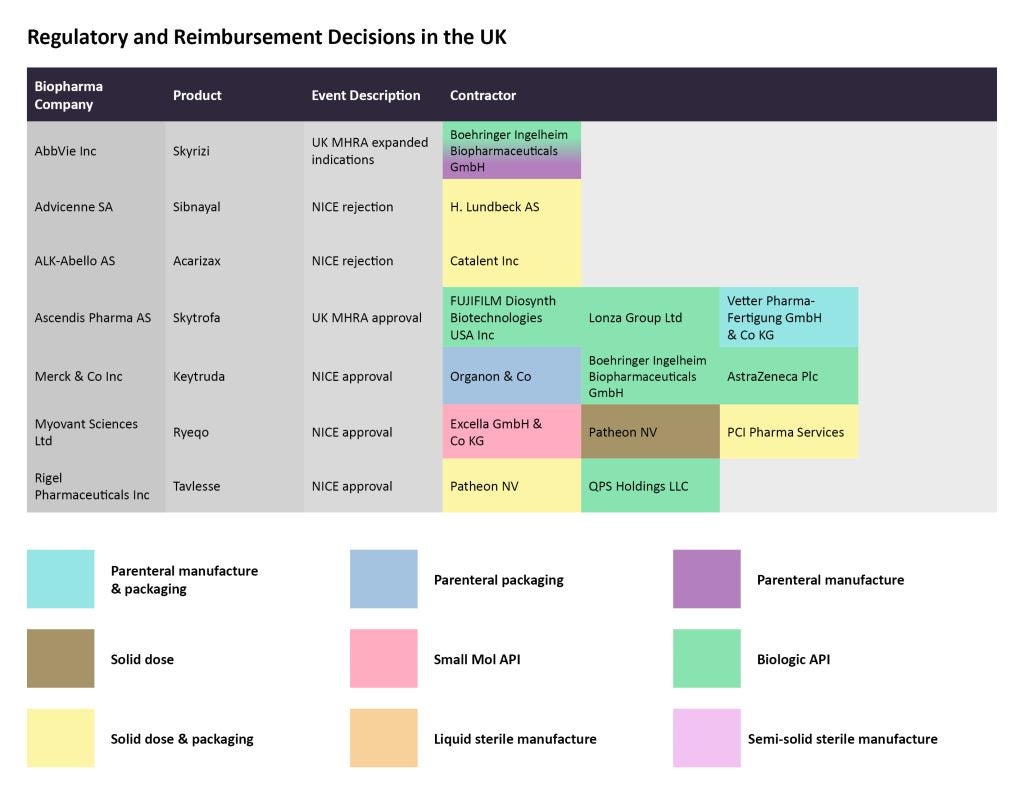
In October, NICE recommended the use of Myovant Sciences’ Ryeqo (regulgolix) for the treatment of uterine fibroids. While Excella GmbH & Co KG is tasked with manufacturing the small molecule API, Patheon, by ThermoFisher Scientific and PCI Pharma Services are contracted for solid dose manufacturing, and solid dose manufacturing and packaging, respectively.
In the same month, NICE did not recommend ALK-Abello’s treatment for allergic rhinitis and allergic asthma caused by house dust mites Acarizax (SQ HDM SLIT), because the company did not provide an evidence submission.
A few weeks ago, the European Commission approved AbbVie’s interleukin (IL)-23 inhibitor Skyrizi (riskanizumab) to treat Crohn’s disease. The MHRA also extended the therapy’s marketing authorization to include a 360mg solution for injection in cartridge version of the therapy in late October. Boehringer Ingelheim Biopharmaceuticals is producing the biologic API and the parenteral manufacturing for the therapy.
To read the previous editions in this series, click here, and here.




