D-Flex® Injection Pen for GLP-1 Medication
Type 2 diabetes is a global health problem. According to expert estimates, the number of patients worldwide will increase by 48% by 2045.

Haselmeier specialises in the development and manufacture of innovative self-injection devices with proprietary designs and technology.
You have successfully submitted your enquiry. Someone from our company will respond ASAP

Haselmeier specialises in the development and manufacture of innovative self-injection devices with proprietary designs and technology.
The company covers all of the steps in the creation of its award-winning devices from design, to planning and industrialisation.
Haselmeier also offers a range of early-stage development activities to ensure all products are high quality. The company conducts human factors studies and consults with user focus groups to provide successful administration. This is combined with a qualified design control process, certified quality system, regulatory expertise, a solid network of partners, and strong manufacturing operations designed to meet and exceed client expectations.
Haselmeier has a world-class design and development team that works in compliance with regulatory requirements. The company creates, designs and industrialises self-injection systems used by pharmaceutical and biotechnology companies worldwide.
Haselmeier creates products to enable a convenient and comfortable experience, which is why patient feedback is integrated into device designs.
Early concepts are prototyped for testing to ensure high-quality products, while human factor studies capture the handling needs and skills of potential users.
This knowledge is integrated into the design of pharmaceutical devices to provide successful administration of drug products and optimal user experience.
Haselmeier offers a range of early stage activities, including:
Development of pharmaceutical devices depends on the understanding of technical, regulatory and operational requirements.
Haselmeier offers integrated design, development and industrialisation services to help clients bridge their serial product into the market.
The company’s qualified design control process, certified quality system, regulatory expertise, network of partners and manufacturing operations are designed to help clients achieve their goals.
Haselmeier’s commercial development and industrialisation services include:
Haselmeier applies the highest standards for manufacturing drug delivery devices to ensure reliable manufacturing and quality processes. The company works with customers to identify product improvements at all stages of its lifecycle, to provide a safe drug delivery device.
Haselmeier provides flexible manufacturing and lifecycle management, including:
Haselmeier develops and manufactures innovative self-injection devices that feature company designs and state-of-the-art technology.
Haselmeier has more than four decades of experience in the field of medical devices. Through the years, the company has built up a top-class planning and development team that is responsible for the design, planning and industrialisation of its innovative self-injection systems. The company manufactures successful products that are used by pharmaceutical and biotechnology companies worldwide.
Headquartered in Switzerland, Haselmeier employs around 200 people worldwide. The company has sales offices in Europe, the US and India. Haselmeier’s products are manufactured in state-of-the-art production facilities in Buchen, Odenwald, Dnešice and Bengaluru.

Type 2 diabetes is a global health problem. According to expert estimates, the number of patients worldwide will increase by 48% by 2045.

The D-Flex injection pen can do more than previous pens. D-Flex can be configured for several fixed-dose values and is thus a variable fixed-dose pen.

Danish pharmaceutical company Novo Nordisk broke ground on its diabetes active pharmaceutical ingredients (DAPIs) production facility in Clayton, North Carolina, in March 2016.

As an innovative, strategic partner company, Haselmeier develops and produces customisd, unique systems that are used worldwide.

Haselmeier and smart injector monitoring and support solutions company Common Sensing have announced a partnership agreement to develop smart connected monitoring and support solutions for users of injectable medicines.

Konrad Betzler was appointed the new chief quality officer (CQO) of the entire Haselmeier Group in October 2017, with responsibility for quality management, quality assurance, and all regulatory matters worldwide.

The Haselmeier D-Flex is a disposable pen for use with 3ml cartridges.

The new Pergoveris® Pen by Haselmeier has received a positive assessment by Committee for Medicinal Products for Human Use (CHMP) at the European Medicines Agency (EMA).

Haselmeier has announced Paul Jansen has been appointed as its senior advisor and member of the board of directors.

Haselmeier has announced that its new product the D-Flex Just one has won the GOOD DESIGN™ Award.

After winning the GOOD DESIGN™ Award and the Red Dot Award: Product Design in 2015, Haselmeier has also been crowned winner of the German Brand Award 2016.

Following approval by the European Medicines Agency (EMA), GONAL-f® 2.0 was launched by Haselmeier and Merck following its original release in 2011.

Haselmeier has added connectivity to its devices portfolio with the announcement of its Axis-D-Connect disposable pen injector.

The Axis-D pen system is a device, which uses 3ml cartridges for variable dose injection.

The i-pen injects standard 3ml cartridges of insulin in variable doses.

The i-pen2 is an injection device, designed for 3ml cartridges.
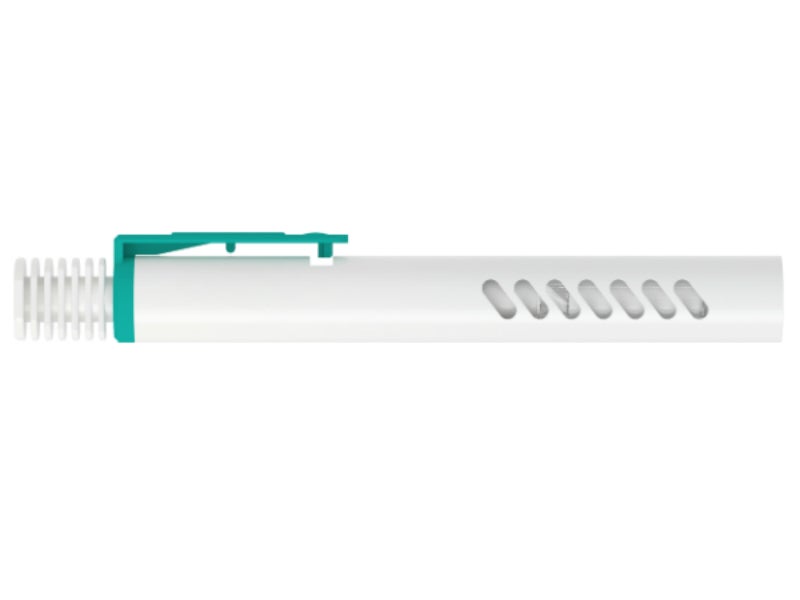
The Penlet is an auto-injector, which is suitable for injecting Enoxaparin or for the treatment of deep vein thrombosis.

The D-Flex is a disposable injection pen that uses 3ml cartridges. It is available in two versions that are designed to cover a freely selectable, regular or irregular dose regimen.