Case Study: Bristol-Myers Squibb
Bristol-Myers Squibb (BMS) is a global biopharmaceutical company headquartered in New York City that specialises in discovering, developing and delivering innovative medicines for patients with serious diseases.

Modality Solutions specialises in the implementation of cold chain management systems for the pharmaceutical industry.
You have successfully submitted your enquiry. Someone from our company will respond ASAP

Modality Solutions is the leading expert in cold chain engineering. We specialise in optimising the biopharmaceutical cold chain for novel, fragile, and controlled-temperature therapies and meeting the rigorous filing requirements for these advanced drug products.
Focusing on the biopharmaceutical, food and life sciences industries, we are deeply committed to safeguarding the integrity and quality of temperature-sensitive products throughout their entire supply chain journey. At Modality Solutions, our unwavering focus is on addressing the unique challenges and requirements intrinsic to these industries, showcasing diverse services and competencies.
Our primary objective at Modality Solutions is to optimise pharmaceutical and biotechnological cold chains, emphasising expediting the regulatory approval process. Our unparalleled success in obtaining approvals within the biopharmaceutical realm is a testament to our distinctive transport simulation lab, multi-disciplinary engineering expertise, and collaborative approach. These factors collectively position us as the preferred partner for addressing the specific cold chain engineering needs of biopharmaceuticals.
With advanced therapies, where regulatory compliance is paramount, Modality Solutions excels in guiding products through the regulatory approval process while optimising commercial cold chains. Our expertise in cold chain engineering, coupled with strict adherence to regulations and comprehensive testing, is crucial for ensuring the success of advanced therapies in the market.
We have a distinguished track record of success in regulatory filings that is complemented by the only contract transport simulation lab of its kind, which showcases our unwavering commitment to innovation and excellence in the field.
Our collaborative and client-centric approach is evident in our role as the trusted cold chain engineering partner for leading pharmaceutical and biopharmaceutical companies. Our expertise extends beyond addressing current industry challenges to anticipating and preparing for the evolving landscape of temperature-sensitive product management. Our proven track record, cutting-edge facilities, and a team of seasoned professionals uniquely position Modality Solutions as an industry leader.
Recognising the evolving landscape of the pharmaceutical and biopharmaceutical sectors, particularly in the context of advanced therapies, we have honed our expertise to meet intricate challenges related to sensitivity to temperature variations and the need for controlled environments. This commitment ensures that advanced drug products can successfully navigate the complex regulatory landscape.
Our commitment to excellence is demonstrated through our emphasis on cold chain engineering, which goes beyond conventional cold chain management practices. It encompasses a holistic and specialised approach tailored to the unique requirements of biopharmaceuticals. We are well aware that success in this field requires a comprehensive understanding of regulatory frameworks, testing protocols, and the ability to adapt to emerging trends.
In summary, Modality Solutions is a beacon of innovation and excellence in cold chain engineering. Our holistic and collaborative approach, coupled with a proven track record, positions us as the go-to partner for pharmaceutical and biopharmaceutical companies seeking to optimise their cold chains. As the industry continues to evolve, Modality Solutions remains at the forefront, ready to meet emerging challenges and contribute to the success of temperature-sensitive products in the global market. Our dedication to excellence and anticipation of industry trends ensure our continued leadership in the dynamic field of cold chain engineering.
Modality Solutions specialises in engineering consulting, offering expertise in designing, implementing and optimising cold chain processes to maintain products within specified temperature ranges. We conduct thorough risk assessments, identify vulnerabilities, and develop strategies to mitigate risks during storage, transportation, and distribution. Additionally, Modality Solutions is committed to assisting clients in navigating complex regulatory requirements ensuring strict adherence to industry standards and guidelines.
With a focus on the biopharmaceutical, food, and life sciences industries, we provide tailored solutions to meet the unique needs of our clients. Through our comprehensive services, Modality Solutions ensures the integrity and quality of temperature-sensitive products throughout their supply chain journey.
Modality Solutions excels in protocol development, crafting customised validation protocols and testing procedures to affirm the effectiveness of cold chain processes. Through performance qualification studies, Modality Solutions ensures the integrity of temperature-sensitive products by verifying their stability throughout the supply chain.
Leveraging advanced data analysis, Modality Solutions offers valuable insights into temperature excursions and deviations, empowering clients to make informed decisions regarding their products. This meticulous approach to data analysis enhances the overall understanding of cold chain processes and aids in optimising performance.
With a commitment to precision and excellence, Modality Solutions plays a crucial role in safeguarding the quality and reliability of temperature-sensitive products for its clients in diverse industries.
Modality Solutions specialises in package design, creating and testing temperature-controlled packaging solutions to shield products from temperature fluctuations, shock, and environmental stresses. Through rigorous thermal testing, Modality Solutions ensures that these packaging solutions maintain the required temperature profiles under diverse conditions.
Emphasising sustainability, we actively pursue eco-friendly packaging options to reduce waste and lessen environmental impact. With a commitment to both product protection and environmental responsibility, we deliver innovative packaging solutions that align with industry standards and promote sustainable practices.
Navigating the maze of regulatory requirements is daunting for many companies, and our team of experts possesses an unparalleled understanding of the regulatory complexities surrounding cold chain management. We ensure our clients comply with these stringent requirements, significantly reducing the risk of regulatory violations and associated penalties. Our commitment extends not only to safeguarding our clients’ reputations but also to ensuring the safety and efficacy of their products.

Bristol-Myers Squibb (BMS) is a global biopharmaceutical company headquartered in New York City that specialises in discovering, developing and delivering innovative medicines for patients with serious diseases.

Competing priorities, changing expectations, cost pressures, sustainability initiatives, emerging markets - these are some of the challenges facing cold chain management professionals every day. Solutions that deliver value in the supply chain and enhance your regulatory reputation are required every day, around the globe.

Regenerative medicine is a novel biomolecular approach to exciting new clinical therapies. Generally, this grouping involves three major therapy applications: cell therapies or stem cell injections; immunotherapy, regeneration by biologically active molecules administered alone or as secretions by infused cells; and tissue engineering, transplantation of laboratory-grown organs and tissues.

Immunomedics (www.immunomedics.com) is a clinical-stage biopharmaceutical company developing monoclonal antibody-based products for the targeted treatment of cancer.

The biopharmaceutical industry is at a pivotal moment, particularly those involved in developing drugs for rare diseases.

Competing priorities, changing expectations, cost pressures, sustainability initiatives and emerging markets ‒ these are some of the challenges facing cold chain management professionals every day.

When a monoclonal antibody therapy advances through its development lifecycle, planning ahead and optimising the biopharmaceutical cold chain is key to advancing towards approval.

Transport simulation is intended to expose the product to the concurrent combination of shock, vibration, temperature and pressure in a controlled and repeatable manner.

And why is Modality Solutions (a cold chain engineering firm) asking this question?

Today's pharmaceutical cold chain network is increasingly complex and involves many players. Competing priorities, changing expectations, cost pressures, sustainability initiatives, and emerging markets - are some of the challenges facing cold chain management professionals every day.

When it comes to ensuring the integrity of temperature-sensitive products during transit, choosing the right thermal packaging is paramount.

It’s common for the FDA to issue an Information Request (IR) during its review cycle and it’s critical that you respond quickly and effectively to get your vaccine or therapeutic approved in a timely way.

Johnson & Johnson’s new $2bn biologics manufacturing facility will be located in North Carolina, US.

Modality Solutions is proud to announce the launch of our new Greenwood Lab—a state-of-the-art facility designed to dramatically reduce lead times and expand our testing capabilities for cold chain logistics.

Four leading life science companies—Suttons Creek, Modality Solutions, CLINTREX, and ToxStrategies—announce their unification under a new brand: BlueRidge Life Sciences.

A Proven Approach to Ensure Success.

Houston-based biopharmaceutical cold chain validation engineering firm Modality Solutions is thrilled to declare it made the Houston Business Journal’s 2020 Fast 100 rankings of the city’s fastest-growing privately held companies for the second year in a row.

Last month, the FDA approved tafasitamab-cxix (official name Monjuvi®) in combination with Lenalidomide as a second-line treatment for adult patients experiencing relapsed or Refractory Diffuse Large B-cell Lymphoma (DLBCL) for MorphoSys, one of our consumers.

The Coronavirus Treatment Acceleration Program launched by the FDA in response to Covid-19 is a Supercharged Fast-Track Approval process that reduces the Fast-Track Approval timeline from 18 months to as soon as possible in order to get treatment to the patients as fast as possible.

Clinical trials around the world are being disrupted by the Covid-19 pandemic, leaving sponsors with no choice sometimes but to suspend some trials and delay others.

Artificial intelligence (AI) is guaranteed to renovate the pharmaceutical cold chain; not in the distant, proposed future, but in the following several years.

Modality Solutions, a leading biopharmaceutical cold chain engineering firm, is pleased to announce it achieved its ISO 9001:2015 registration by certification body Intertek.

Biosimilars have been a point of contention within the pharmaceutical industry for the past 30 years.

Modality Solutions specializes in integrating cold chain operations, developing transport validation strategies, supporting global regulatory applications, and executing global clinical trial operations.

2019 saw a resurgence of antibody-drug conjugates (ADCs) with seven new approvals and close to 100 investigational ADCs currently in pre-clinical and clinical trials.

With five FDA programmes moving drugs through approval to commercialisation, the speed of scale-up is quickening, shortening time to market by months.

Validating your cold chain to meet and exceed increasingly stringent regulatory expectations in today’s global market is a challenge. With more than 35 international cold chain regulatory compliance guidance documents, global standards are becoming more comprehensive and increasingly complex.

Modality Solutions, a biopharmaceutical cold chain engineering firm, is pleased to announce it made its first appearance on the Houston Business Journal’s Fast 100 list. Modality Solutions ranked No 91 with reported two-year revenue growth of 56%.

Biopharmaceutical cold chain engineering firm Modality Solutions is pleased to announce it ranked No 3094 on Inc Magazine’s annual Inc 5000 list, one of the most prestigious rankings of the nation’s fastest-growing private companies.

Over the course of the last decade, the use of drones has been trialled for use in expanding the ability of supply chain professionals to deliver temperature-sensitive drug therapies and life-saving vaccines.

Modality Solutions’ President Gary Hutchinson has been accepted into Forbes Technology Council, an invitation-only community for world-class chief information officers (CIO), chief technology officers (CTO) and technology executives.

Temperature data loggers have been around for decades and were one of the first sensors in the biopharmaceutical cold chain. This technology provided temperature audit trails of shipments in transit, enabling partners in the cold chain to confirm whether the products remained within specification throughout transit and verifying the acceptability of the product for the next partner in the cold chain.

Modality Solutions is a biopharmaceutical cold chain engineering firm with regulatory filing authorities, logistics network experts, and experienced integrated staffing professionals.

Immunotherapies are an increasingly complex set of treatments for a wide variety of diseases and medical conditions.

Modality Solutions has announced it will be a silver sponsor and exhibitor at Executive Platforms’ Biomanufacturing World Summit (BMWS).

Full-service engineering firm Modality Solutions offers drug product formulation testing with transport simulation for drug product formulations to concurrently expose drug products to temperature, shock, vibration, humidity and pressure, as they occur during real-world distribution.

Modality Solutions is participating as both a Silver Sponsor and exhibitor at this year’s Executive Platforms’ Biomanufacturing World Summit (BMWS).

Modality Solutions cold chain experts recently published and made available its new Cold Chain Process Validation Guide.

Cold chain experts Modality Solutions have published and made available for free its new white paper, ‘Drug Product Formulation Testing with Transport Simulation’.

Robert Battista is a consulting engineer at Modality Solutions.

What is the impact of the cycled setting? Modality Solutions has results to share. While running a series of controlled tests for a client, the temperature control unit (TCU) was inadvertently set to cycle mode allowing the company to compare and contrast the results of a continuous versus cycled setting.

Modality Solutions’ president Gary Hutchinson and principal Daniel Littlefield will be attending and participating in the American Biomanufacturing Summit.

Modality Solutions has announced the transformation of Modality-Solutions.com into an engaging, content-rich, all-device compatible website.

Modality Solutions has announced that it will be sponsoring and presenting at the 15th Annual Global Forum - Temperature Controlled Life Science Supply Chains.

Modality Solutions has hired Robert Battista as consulting engineer, expanding its team of cold chain management experts.

Modality Solutions has announced its sponsorship and roundtable leadership at the 17th Annual World Vaccine Congress (WVA) in Washington, DC.

Integrated cold chain management solutions provider Modality Solutions has announced its sponsorship and presentation at this year's Global Cold Chain Exchange.

Modality Solutions is pleased to announce its bronze sponsorship and panel participation at the 9th Annual Bio Supply Management Alliance (BSMA) Forum titled 'Preparing the Biotech Supply Chain for 2025, The Onward March'.

Cold chain management solutions company Modality Solutions has announced it will sponsor one of the largest events for temperature controlled life science supply chains, 14th Annual Cold Chain Management Logistics Global Forum.

Modality Solutions has hired Andrew Larrigan as consulting engineer, which involves writing proposals and reports for a distribution and thermal testing.

Life-sciences contract service provider BioConvergence has rebranded as Singota Solutions to respond to its client's needs.

The role of Technical Consulting Engineer at Modality Solutions has been given to Hannah Anderson, who is currently helping to provide company’s pharmaceutical clients with program management of its thermal package engineering services.

Modality Solutions is pleased to announce that president Gary Hutchinson will be a panelist at the 16th Annual Trans-Pacific Maritime (TPM) Conference in Long Beach, California from 28 February to 2 March.

Transportation hazards affect drug product quality and efficacy.

Modality Solutions operates our Advantage Transport Simulation Laboratory™ that has been continuously validated to current GMP (Good Manufacturing Practices) since its installation in 2012.
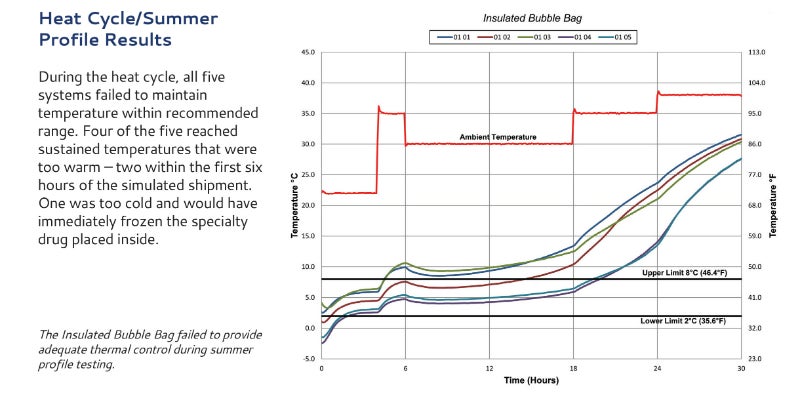
The need for a standardized package qualification process was recognized by the International Safe Transit Association (ISTA) in April 2014. Modality Solutions partnered with ISTA and spearheaded this standardization process.

Modality Solutions is the leader in clinical trial operations for vaccines for infectious diseases in emerging markets because we understand the challenges healthcare staff experience with proper storage and handling risks associated with temperature-controlled vaccines and biologics.

Working with Modality Solutions gives clients the distinct advantage of having three firms integrated into one.

Carson Dickey from Modality Solutions speaks on the use of heat transfer concepts to evaluate pre-qualified thermal shippers in biopharmaceuticals.

Modality Solutions' Carson Dickey and Andrew Larrigan discuss the opportunities presented by information requests and how to respond to them.