Smart Building
Implement social distancing policies with real-time feedback to ensure the health and safety of workers, and analyse logs to identify high-risk behaviours.

Nymi™ delivers a wrist-worn platform that seamlessly integrates employees with their physical and digital networks to address issues in security, compliance, productivity, and user experience in one solution.
You have successfully submitted your enquiry. Someone from our company will respond ASAP

Nymi makes work secure and frictionless for organizations and their employees with a wearable connected worker platform.
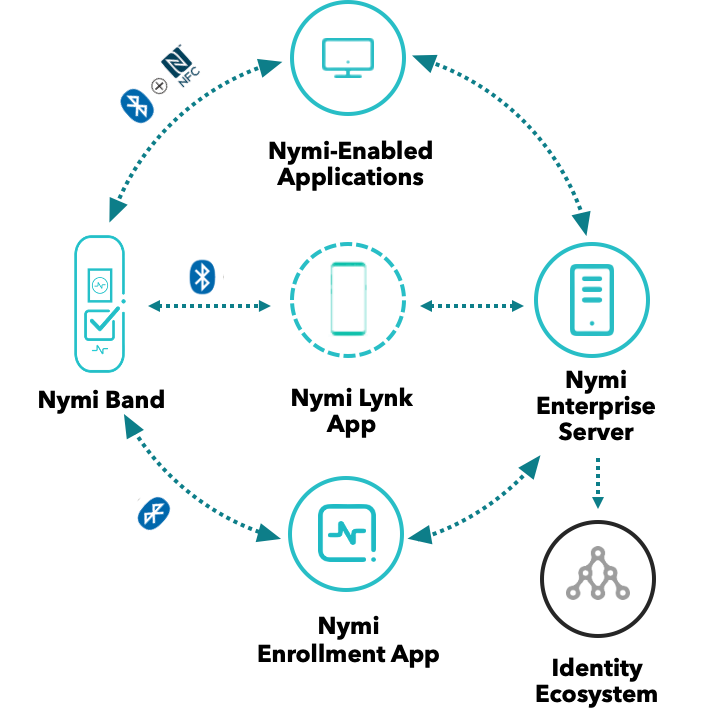
The Nymi Connected Worker Platform (CWP) has created a world where employees can fully integrate with their physical and digital networks in a manner that is safe, secure, and simple. A connected workforce is continuously authenticated throughout their workday. This provides employees with a seamless employee journey without the burden and chokepoints of traditional credentials, such as passwords, pins, key cards, and fobs.
The Nymi CWP shifts the burden of connectivity onto a secure workplace wearable enabled by fingerprint, heartbeat, and continuous On-Body Detection. The Nymi BandTM blends biometrics, cryptography, and multifactor authentication in a Zero Trust framework to provide a continuous, secure, and private link for employees to all their workplace systems in one authentication. A connected workforce strengthens security, compliance, privacy, and productivity even in the most challenging environments, as proven in its deployment across 9 of the top 10 global pharmaceutical companies.
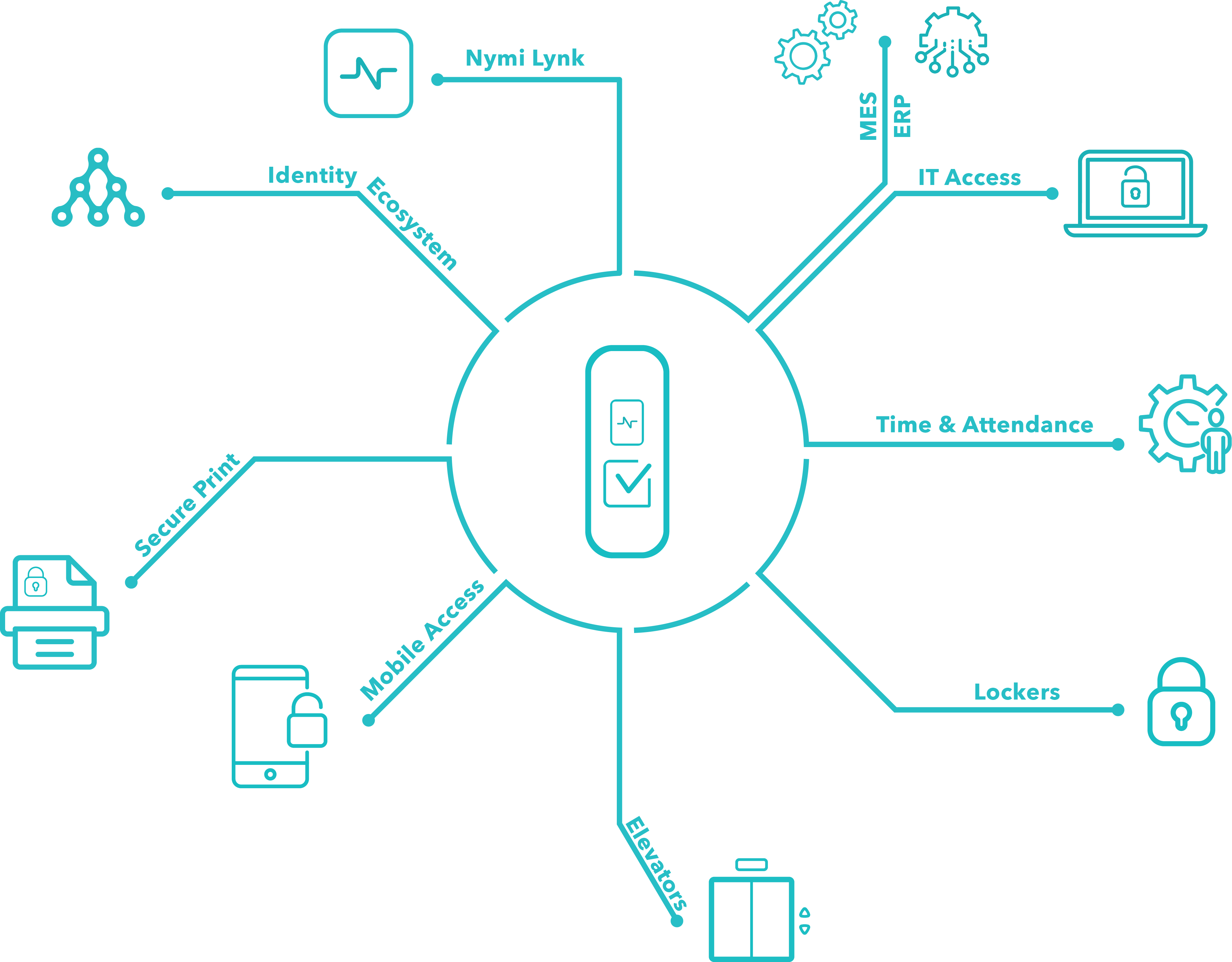
The Nymi CWP is an enterprise wearable solution that is delivered through a connected worker service. It is designed for easy deployment through a vast partner ecosystem, standards-based integrations, and pre-validation packages.
Operator logins and e-signatures are a significant area for productivity improvements. These time-consuming, repetitive actions not only hinder production, but interrupt operator workflow and experience. The Nymi Band offers multifactor, biometric, and continuous authentication to replace the need for passwords and paper signatures. Logins are replaced with intuitive, intent-based actions that take a few seconds. This increases operator productivity, user satisfaction, and security.
The time saved by reducing login times and removing user authentication errors (failed passwords) leads to dramatic increases in cost savings and efficiency for manufacturing environments.
The Nymi Band’s natural UX also delivers full functionality under Personal Protective Equipment (PPE). Operators can be dressed and protected in gloves, cleanroom garments, clean suits, etc. and use their Nymi Band normally.
The Nymi Band’s design inherently increase’s a company’s security.
This innovative approach to connecting workers allows employees to authenticate to their Nymi Band as little as once per shift activating it for seamless and secure use until the operator takes it off.
The Nymi Band provides ease of use and ease of mind with its UX design, as well as its privacy framework. Personal biometric information is secured locally on the band and never transmitted to a server or the Cloud. Nymi’s secure, independently reviewed architecture and hardware ensures tamper-resistant cryptographic processes that protect confidential data and certificates.
Nymi’s connected worker approach to authentication also supports improved compliance with international regulatory requirements.
Only specific employees can perform certain tasks, at the required times and for the right reasons. By offering improved data integrity and a reduced risk of compromised credentials, Nymi can assist with critical requirements of the US Food and Drug Administration (FDA) 21 CFR Part 11 and EU GMP Annex 11. Through the implementation of technology, in addition to policies and procedures, the system will meet regulations and maintain a compliant ecosystem.
Nymi also provides real-time, on-demand traceability and secure electronic records, allowing for simpler and less intrusive audits.
Nymi understands that manufacturing environments require massive investment, so its service supports the move to digitization and reduces friction in the use of MES. The Nymi CWP seamlessly integrates with existing enterprise IT, ERP, SSO and identity management solutions for ease of deployment with minimal changes to IT and operational environments.
The company’s security architecture can be integrated with MES in highly regulated production industries to remove the burden of authentication from the user, while increasing accountability and eliminating failed logins.
Nymi has developed partnerships with industry leaders such as Evidian and Werum, allowing Nymi to access multiple software systems with a single login and with no custom integration required.
Nymi is a Toronto-based tech company that exists to create a world where people and technology converge in a manner that is safe, secure, and simple. We enable businesses to achieve true digital transformation by empowering the workforce with a wearable platform that allows them to connect confidently in an increasingly digital world.

Implement social distancing policies with real-time feedback to ensure the health and safety of workers, and analyse logs to identify high-risk behaviours.

Increasingly, organisations are integrating workplace wearables to help drive business objectives and operational efficiencies.

Every day, we are developing more and more sophisticated technology, but still connect to these systems in antiquated ways that have proven issues around security, privacy, compliance, productivity, and user experience.

Nymi Band™, the standards-based workplace wearable, is now certified as a FIDO2 authenticator. As a member of the FIDO Alliance since 2014, Nymi designed the solution to be compliant with FIDO2 from the ground up and has always been committed to the use of safe, secure and simple authentication. This certification further deepens that commitment.

Nymi has published the results of a questionnaire into US consumers’ attitudes to wearing employer-issued technology that could enable them to stay safe and drive productivity as businesses prepare for hybrid workplaces.

rf IDEAS, a leading manufacturer of credential readers for authentication and logical access, announced a new technology partnership with Nymi, the creator of the Nymi Band™, an innovative workplace wearable wristband for secure hands-free authentication.

Nymi, Inc. today announced an integration with Tulip to provide secure, seamless, biometric authentication in Tulip Apps through the Nymi Band™.

Nymi, Inc and ELATEC, a world leader in RFID readers and NFC/BLE mobile device readers, are pleased to announce the commencement of a technology partnership.

Nymi, Inc. announced today, customers looking to leverage the Ping Intelligent Identity platform can now use the Nymi Band™, a biometric-enabled multi-factor authentication wearable that supports FIDO2 for passwordless authentication.

Nymi, Inc is pleased to announce that it will be teaming up with Quuppa to utilise the latter's Real-Time Locating Systems (RTLS) to drive innovative industry applications for the Nymi Band™.

Applying learnings from Y2K to navigate the future of a pandemic-influenced landscape.

Nymi, Inc has formally announced its technology partner programme to address companies’ increasing and evolving needs to connect their active workers in a modern, secure and safe way.

A global leader in advanced identity solutions, HID Global, is pleased to declare that its next-generation Seos® credential technology will permit Nymi Band 3.0 operators to flawlessly open doors, as well as verify to devices, machines, and systems.

Nymi is pleased to announce the release of its Version 3.0 workplace wearable wristband, now allowing multiple industries to unite Nymi’s password-less technology with applications that guarantee the health, safety and security of associated workers.

Today’s Pharma employee is highly trained, experienced and outfitted with innovative tools to imagine, strategise and manufacture tomorrow’s solutions. However, they’re hindered by yesterday’s infrastructure.

Werum IT Solutions is pleased to announce the launch of the biometric authentication solution K.ME-IN with Nymi, for integration within Werum’s PAS-X Manufacturing Execution System (MES).

Xyntek Incorporated, a world leader in pharmaceutical and life sciences automation and IT solutions, has signed a partnership agreement with Nymi to increase their Turnkey Biometrics Authentication Platform to incorporate wearable technology.

Balancing and finding productivity in opposites. In companies, compliance and security are two poles moving away from one another.

Nymi is excited to welcome Chris Sullivan as its new Chief Executive Officer.

Nymi has partnered with Rockwell Automation’s ThinManager, a global leader in the automation industry and provider of full-feature centralised thin client and Remote Desktop Server management software.

The demand for increased pharmaceutical manufacturing is only offset by the growing stringent requirements by regulatory bodies for good governance over the manufacturing process. Warning letters for data integrity issues have more than doubled in the last couple of years. The need for trackability and traceability is at an all-time high.

A fully digital and smart workplace offers many benefits, including lower costs, improved quality, and higher productivity. Nearly everyone agrees about that. Yet in pharmaceutical manufacturing, the pace of change has been slow.

Highly regulated manufacturing operations must control access to devices, applications and physical spaces to ensure data integrity, compliance and security without compromising productivity. Nymi is bringing a new innovative authentication solution to these operations with the Nymi Enterprise Edition.

Nymi has announced that it has entered a strategic partnership with Konica Minolta that includes a financial investment by Konica Minolta in Nymi.

Nymi band is a workplace wearable that enables employees and processes to stay safe and secure; the product is handsfree and straightforward to operate.
Healthcare industries struggle to deliver the advantages of digital transformation (DX), as commonplace solutions entail trade-offs between security, compliance, and operator complacency.
Developed to nurture an ecosystem that digitises the workforce, Nymi unites a complete set of benefits aimed at onboarding teams quickly through the Nymi Technology Partner Program.

Nymi has implemented a Quality Management System (QMS) that aligns with our customer requirements and guides the development of products and solutions.